Documents
UW Madison Lab Accident Reports to NIH
Nov. 1 2022 — 11:45 a.m.

~,_,t.1rac,,,_~
( - National Institutes of Health .. ;~~\.-
December 24~ 2013
redacted by agreement I
Associate Vice Chancellor for Research Policy
University of Wisconsin
327 Bascom HalJ 3
500 Lincoln Drive
Madison, WI 53 706-1380
Dearfedacted by agreement
U.S. Public Health Service
Bethesda, Maryland 20892
Office of Biotechnology Activities
National Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda. MO 20892-7985
(301) 496-9838 (Phone)
(301) 496-9839 (Fax)
• hllp:/loba.od.nih.gov/
We are writing in reply to your correspondence of December 20. 2013, that responded to two
letters from the National Institutes of Health (Nil-I) Oflicc of Biotechnology Activities (OBA),
dated December t 6, 20 I 3. These two letters addressed two separate incidents involving
recombinant DNA research with highly pathogenic avian influenza HPAl) HSNJ that have
occurred recently in the ABSL3+ laboratory ot edacted by agreement
In our December 16, 20 I 3, letters we detailed our concerns regarding the University of
Wisconsin's Jack of a dedicated quarantine facility to house individual_s who have experienced a
high-risk exposure to mammalian-transmissible strains of HPAI HSN I. We also conveyed our
concerns regarding specific biosafety practices associated with these incidents.
After reviewing the information you have submitted, NIH OBA has determined that the
University of Wisconsin has adequately responded to our concerns. Detailed infonnation
regarding your response appears below.
Lack of a 1/edicated quarll11li11e facilitv
Jn our December 16,2013, letter regarding the ncedlestick incident, we stated that to be
compliant with the NIH G11ideli11esfor Research lnvolvi11g Recombi11a11t or Sy111hetic Nucleic
Acid Molecules (Nllf Guidelines), the University of Wisconsin must find a dedicated quarantine
facility outside of the individual's permanent residence (I) in which an individual e.xposcd to
mammalian-transmissible HP Al H5N 1 can be salcly isolated for up to IO days, and (2) that can
be decontaminated easily after the individual's depai1ure.
In your response, you stated that the University of Wisconsin will utjlizc the University of
Wisconsin Hospital as a quarantine facility in the event of a high-risk exposure to mammaliantransmissible strains of HSN!. Your response also included an updated standard operating
procedure (SOP) that reflects this change. •

redacted by agreement
December 24, 2013
Page 2
Cm1L'l!ms rel"tiug to bio.wfetr pmclices
Both of our December 16, 2013, letters conveyed our concerns regarding the biosafoty practices
associated with each incidcnl. Spccific,11ly, we were eonccmc<l that a researcher's personal
protective equipment did not cover his ankles while in the ABSL3+ laboratory, creating an
opportunity for dermal exposure lo hazardous agents. We were also concerned that a researcher
had used a needle to collect lissuc culture supernatant in violation of the University of
Wisconsin's own policies, which 011ly pcnnit needles to he used in the ABSL3+ laboratory to
anesthetize research animals, drnw blood from research animals, or inoculate eggs.
With regard to the issue of bare skin, your response slates that the principal i nvcstigalor will
require that high-top booties be worn umkrncath the researcher's tyvek suit and inside of the
dedicated clogs. ·n1e dogs will then be covered with regular shoe covers. This change has been
incorporated into the SOPs for the laboratory, and you have provided OBA a copy of that SOP.
We also understand from your letter that you arc rcgucstin~ clarification regarding the issue of
exposed skin in relation to Purified Air Pmvcrcd Respirators, which \Ve will respond to in a
separate communication.
With respect to the nccdlestick incident, your response states that the University has conducted
two training sessions since this event. The first session was aimed al all current and future
biocontainment laboratory researchers and was led by a senior scientist at the University of
Wisconsin. The second session ,vas led by two senior scientists, and the slides from that training
event have been included in your response. 1\dditionally, the SOPs for sharps use in lhc
ABSL3+ laboratory have been revised to be more specific. The new SOP articulates that sharps
will only be used when administering drugs to an animal or drawing blood from an animal.
When either of these procedures are being performed with mammalian-transmissible influcn7..a
viruses, two individuals will be required to be present for the procedure.
With respect to the issues above, the University of Wisconsin response is now t:onsistcnt with the
requirements of the NIH Guide!;nes. which arc a term and condition of the funding or this project.
As such, the resumption of research involving mnmmalian-transmissiblc strains oflI5Nl is
appropriate as our biosafety concerns have been addressed. In the meantime, i r you have any
questions about this letter, please Ice! free to co11tacl us.
s· ly,
. uelinc ., M.D.
i Cling Di
})nice of Biotechnology Activities
V

redacted by agreement
December 24, 2013
Page 3
cc: Redacted by agreement
Capt. Robbin Weyant, Ph.D., Director. Division of Sc Ice\ Agents and Toxins. CDC
Freeda E. Isaac. D.V.M., Director, Agriculture Select Agent Program, USDA /\PHIS
Amy P. Patterson, M.D., Associate Dircclor for Science Policy. Nll I
Sally Rockey, Ph.D .. Deputy Director for E.xlrmnural Research, NII I
Mary Kirker, Program Director, Grants Management Program. NIAID, NIH
Matthew Fenton, Ph.D., Director, Division of Extramurnl Activities, NIAID, Nil-I
Victoria Connors, Hranch ChicL Grants Management Program, NIAID, NIH
Teresa Haugucl, Ph.D., Program Offic~r. NIA ID. NI 1-1
Dinne Dean, Director. Division of Grants Compliance and Oversight, OER, NIH

December 20, 2013
Jacqueline Corrigan-Curay, M.D., J.D.
Acting Director
Office of Biotechnology Activities
National Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda, MD 20892
Dear Dr. Corrigan-Curay:
TH£ UNIVERSITY
----q/----
WISCONSIN
MADISON
Thank you for the opportunity to respond to your letter dated December 16, 2013 regarding questions
NIH OBA has about an incident at our institution where a researcher used a needle inappropriately and in
the process acquired a needle stick.
In response to this incident, there have been two training sessions so far to discuss this event. The first
was a questions and answer session on November 19, 2013 involving all current and future
biocontainment laboratory researchers. This was directed by a senior scientist and the University of
Wisconsin Select Agent Program's trainer. They discussed the use the sharps in the containment labs. The
second training session occurred on December 18, 2013 and was led by two senior scientists and the
Select Agent Program trainer. The training materials are provided with this memo. In summary, the
sharps standard operating procedure (SOP) has been revised to be more specific and modify current
procedures to make them safer. Sharp needles will only be used for administering drugs to animals and
drawing blood from animals. When either of these procedures are being done with reconstructed 1918
influenza virus or mammalians transmissible influenza viruses, two people will be required for the
procedure. When inoculating eggs, only blunt or dispensing needles will be allowed. Lastly, the SOP was
also updated to include the appropriate cleaning of necropsy tools.
In regards to the second matter described in your letter, we have determined that your suggestion of a
hospital isolation room at the University of Wisconsin Hospital is the best option to comply with the
requirement to have a dedicated facility outside of a personal residence to quarantine. The University
spent a significant amount of time enhancing our influenza exposure control plan in 2012. At that time,
the University, our health care providers, and the Department of Public Health debated the best place for
quarantine and determined that complications could arise with hospital quarantine. Based on these
complications, they felt a home quarantine was appropriate for all exotic influenza viruses. When
consulted about NIH OBA's requirement, our health care providers reiterated concerns but they have
agreed to provide this service for us so our program is in compliance with the terms and conditions of the
grant. Attached are the revised exposure control plan and exposure SOP reflecting these changes. The
changes are effective immediately.
Graduate School
Bascom Hall University of Wisconsin-Jiaclison 500 Lincoln Drive :Madison, W'l 53706-1380
Dean's Office
608-262-1044
Fu:: 60S/262-5134
Graduate Admissions & Academic
Sen-ices, Diversity Resources
608/262-2433, F:ax: 608i265-9505
Accounanc
608/:262-5835
Fu: 608/262-5134
Human Resources
608/262-5802
Fu: 608/262-5235
P.rofessio.nal De-.,eJopment
& Engagement
~S/262-2433,. Fu:: 608/262-5134

Jacqueline Corrigan-Curay, J.D, M.D.
December 20, 2013
Page 2
It should be noted no work has been performed with mammalian transmissible H5Nl viruses at the
University of Wisconsin-Madison since the start of the moratorium in January 2012. Currently, there are
no plans over the next several months to resume experiments with these viruses. University
administration will be informed before the experiments with mammalian transmissible H5Nl viruses
resume.
Please contact me if you have any questions or concerns.
Sincerely,
Redacted by agreement
Associate Vice Chancellor for Research Policy
Attachments: IRI Sharps SOP
Incident Follow-up Training Materials - November 19, 2013
Incident Follow-up Training Materials - December 18, 2013
IRl Exposure Plan (Overview)
IRI Exposure Plan (SOP)

IRI SOP# 18
Sharps
Injury from sharps is a significant concern in the BSL-3 laboratories. All work must be done in a
manner that reduces the risk of sharp injuries. Sharps are laboratory items such as needles, scissors,
and broken glassware. This SOP will list the precautions that must be taken when working with
sharps.
Needles
Careful handling of needles is very important. Used needles must not be bent, sheared, broken,
recapped, removed from disposable syringes or otherwise manipulated by hand before disposal. The
used syringe with needle must go directly into a sharps disposal container. The sharps disposal
container must be located at the work site. Do not overfill the sharps disposal containers. Once
used syringes and other sharps reach the "fill-line", properly dispose of the container and start a new
container. When possible, the use of safety glide needles is recommended when working with
animals.
Sharp needles should only be used for the following:
administrating drugs to animals
drawing blood from animals
If the procedure is being performed on an animal infected with a recombinant virus with one
or more genes from the 1918 virus or a mammalian transmissible influenza virus of avian
origin, the procedure must be performed in the presence of another researcher.
For the inoculation of eggs and harvesting virus from eggs, only dispensing needles are
allowed.
Necropsy tools
Scissors used with forceps should be the primary tools used to perform necropsies.
Instruments should be carefully and properly cleaned using a brush and stored in properly
labeled, hard-walled containers when not in use.
If necropsies are performed on animals infected with a recombinant virus with one or more
genes from the 1918 virus or a mammalian transmissible influenza virus of avian origin, the
procedure must be performed in the presence of another researcher.
Broken Glassware
Every effort must be made to minimize the use of glass in the BSL3 suites. Plastic containers and
bottles must be substituted whenever possible. In the event that glass is broken, it must not be
handled directly. Instead it should be picked up with tongs, forceps, or a broom and dustpan. Broken
glass must be placed in a sharps container for autoclaving and disposal.

IRI Incident Follow-up Training November 19'", 2013
Trainers:
Influenza Research Institute
UW Environment Health and Safety
Select agent researchers in Yoshi Kawaoka's laboratory attended a meeting on November 19th in
response to two laboratory incidences that had recently took place in the containment labs of the IRI, a
biohazardous spill, and a needle stick.
- started the training session with an interactive power point regarding risk assessment and risk
mitigation. As a group researchers were asked to list what the risks are for conducting the following
three tasks:
• Entry into a BSL-3 suite
• Emptying the BSL-3 trash
• Inoculating eggs in the BSL-3
Then researchers were asked for ways in which they mitigate the risks associated with those activities.
- asked researchers to always ask themselves "is what I am doing safe?" and to be consciously
thinking about all of their actions in BSL-3 also reiterated that if you are unsure or do
not know what you are doing, ask someone before proceeding, and if you are doing a procedure that
you have never done before in BSL-3 containment, you must discuss this procedure with -
- before starting the research.
The use of sharps in the BSL-2 and BSL-3 was reviewed. Each researcher was asked to fill out an
assignment with the following questions on it:
• Do you use disposable sharps (e.g., syringe needles, razor blades) for any of the research you
perform in either the BSL-2 or BSL-3 laboratory?
• If yes, please list the types of procedures that require sharps use.
• Describe the methods you use to reduce the danger associated with sharps in your research.
Researchers shared ways they mitigate risk when handling sharps in the BSL-3 containment laboratories.
finished the session by discussing the biohazard spill incident. The researcher involved in the spill
incident was asked to talk the group though exactly what happened and demonstrate was
holding the plates when the incident occurred. Following the reenactment the researcher was
interviewed a bout different aspects of the incident so that researchers cou Id learn firsthand what it
might feel like to have an accidental spill. Here is a sampling of the questions asked:
• Did you carry the plates any differently that day than you normally do?
• What was the first thought that came to your mind when you dropped the plate?
• Did you consult the spill protocol?

• What was the hardest or most challenging part of the spill clean-up process?
• Did you have all of the spill supplies available and in the room with you for clean up?
• Did you ever have a moment when you panicked?
• What was running through your head?
• What was your worst fear? Quarantine?
• At what point did you contact your family? Were you updating them as things went along or did
you wait until the final decision was made? Why?
• What was the worst part of this process?
• Is there a part of the process that you would change?
• Is there anything that you would do differently?
After the discussion researchers were reminded to take care of themselves both physically and mentally
and that if they arc having an off day or feel like they are not fit to work in the laboratory they should
say something and make arrangements to have a partner in the laboratory with them or arrange for
someone else to take over their project.

Risk assessment and risk mitigation
apply to every single thing you do in the
BSL-3. laboratory.

Risk assessment: What are the dangers
associated with each action you perform?
Example: entry into a BSL-3 suite
Example: emptying the trash
Example: egg inoculation

Risk mitigation: How do you protect
yourselfrom the dangers associated with
a particular activity?
Example: entry into the BSL-3 suite
Example: emptying the trash
Example: egg inoculation
Always ask yourself:
Is what I am doing safe?
. If you don't know:
Ask someone before proceeding·

If you are performing aprocedure you've
never done before in BSL-3,
... you must discuss this procedure with a
senior scientist before starting.
This applies even to procedures you
have performed many times in BSL-2.

Sharps Training 12/18/2013
Points to Cover:
Stress that sharp needles may only be used for drug injections or blood draws. Indicate the needle must
never be manipulated once on the syringe.
Infection of eggs:
NO SHARP NEEDLES; only dispensing needles
Do not hold egg in your hand; keep in egg holder
lnjectables:
Don't recap needle, use holder
Work in pairs if animal is infected with certain viruses*
Blood draws:
Use of safety-lock needles:
Work in pairs if animal is infected with certain viruses*
Necropsies:
Work In pairs
Spread the work out if possible to avoid fatigue
Avoid scalpels
Use forceps to keep fingers away from sharps
Work in pairs if animal is infected with certain viruses*
Use a brush to dean tools
Store tools in a hard-walled, labeled container when not in use. Keep the container in an easily
visible location.
Disposal of sharps inside the biosafety cabinet:
Always keep a sharps container inside the BSC
Do not overfill
Do not place hands inside the opening of the sharps container
Pull disinfectant into needles before dropping them into a sharps container. Close the container
before removing from the BSC
* Recombinant viruses with one or more genes from 1918 virus or transmissible HSNl or H7N9 viruses

Changes to the Use of
Sharps
SOP #18

Purpose of this training:
1) Reduce the use of sharps in the lab
2) Prevent accidents when sharps are u5
by modifying our practices

Areas Identified Where Sharps are Used

Areas Identified Where Sharps are Used
Propagation of virus in eggs
Injectable drugs
Blood draws
Necropsies

Areas Identfied Where Sharps are Used
Propagation of virus in eggs - eliminate
Injectable drugs - modify
Blood draws - modify
Necropsies - modify

Areas Identified Where Sharps are U
Propagation of virus in eggs - eliminate
Areas Identified Where Sharps are Used
Propagation of virus in eggs - eliminate
All sharp needles have been removed
from the 1918 lab and the HS lab. • ! -· .- - -
l
Do not bring any sharp need I 1
into these BSL-3 labs.
·,.1·,
.. :~~· \ .. ,<--·,
'.ih
Areas Identified Where Sharps are Used
Propagation of virus in eggs - eliminate
The use of dispensing needles is manda·
• [
Both in BSL-2 and BSL-3, lfi
1i
l
!I
.,,,
1
.
~, ~-;;_._. ·i~
Areas Identified Where Sharps are Used
Propagation of virus in eggs - eliminate
The use of dispensing needles is manda·
If the gauge or length needs to be
changed, please email
Areas Identified Where Sharps are Used
Propagatio·n of virus _in eggs - modify
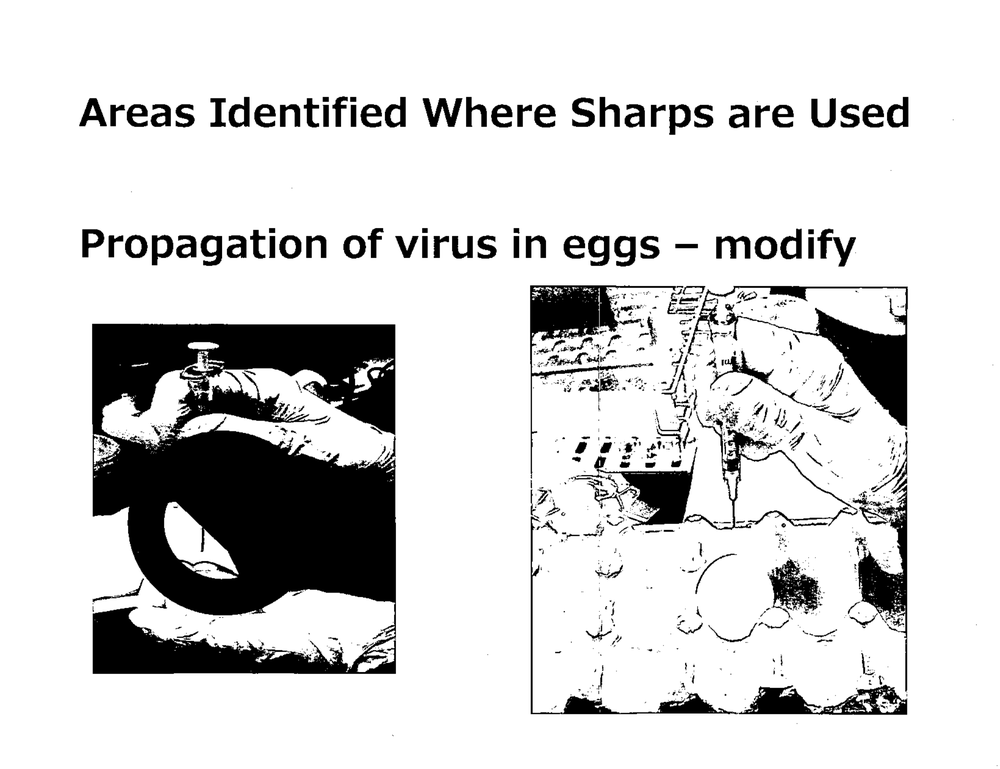
'C
en
aJ
aJ
::::,
ro
i..
0.
en
ro
i..
Ul
.c
aJ
i..
aJ
'C
3:
.c
·-
aJ
C
·-
,1,J
I+--
aJ
en
.....
'C
<t
i..
aJ
ro
E
0
'C
·-
C\
C\
en
I
>
·-
·-
C
aJ
·-
,1,J
0
C
0
l+--
c..
i..
0
0.
ro
C\
ro

Areas Identified Where Sharps are Used
Propagation of virus in eggs -
Any questions ?

Areas Identified Wher~ Sharps are Used
Injectable drugs and blood draws

Areas Identified Where Sharps are Used
Injectable drugs and blood draws
- Sharp needles are required for these
procedures
- Modifications to be safer

Areas Identified Where Sharps are Used
Injectable drugs
Modifications to be safer
Know where your needle is -
Needle stand
Do not· recap ;;
I
r
.... , ..
'l.:)~:;-...... •,
J\_ •..

Areas Identified Where Sharps are Used
Blood draws
- Modifications to be safe·r -
I
SafetyGlide Needles

Areas Identified Where Sharps are Used
Injectable drugs and blood draws
Keep fingers, hands, etc. away from
the sharp end of the needle.

Areas Ide·ntified Where Sharps are Used
Injectable drugs and blood draws
Any questions ?

Areas Identified Where Sharps are Used
Necropsies

Areas Identified Where Sharps are Used
Necropsies
- spread out the work
- avoid scalpels
- use forceps, not your fingers to pull/
RARC will set up training
in Jan/Feb

Areas Identified Where Sharps are Used
Necropsies
- maintenance of necropsy tools
Brushes to clean after use
Hard-walled, labeled containers

Buddy System
For any procedure involving sharps and
any virus, it is recommended to have a
research partner to assist.

Buddy System
For any procedure involving sharps and
any virus, it is recommended to have a
research partner to assist.
- BSL-3 animal lab tech
Buddy System
It is REQUIRED to have another researcher
present and assisting when sharps are being
used when working with
Transmissible avian influenza viruses
Recombinant virus with 1 or more genes
from 1918 virus

Disposi'ng of Sharps
Immediately dispose of used sharps in a Sharps Container
DO NOT OVERFILL

General Summary
We need to communicate more
Think about our actions
Stay focus
Cannot have more accidents
But MUST report any incidents ,
even the most minor
Sharps Summary
No sharps in the BSL-3 labs
Dispensing needles for eggs
Needle stands, SafetyGlide needles
Working with a partner
Do NOT overfill the used sharps container

Where do we go from here
RARC animal training
One-on-one training for sharps
Standard virus propagation in eggs training
IRI SOP #48
Exposure Plan - Influenza Viruses
At the Influenza Research Institute (IRI) the following influenza viruses are used in our BSL-3+ and BSL-3
Agriculture (Ag) laboratories:
- Highly pathogenic avian influenza viruses such as H5N1 viruses
- Human H2N2 viruses
- H1N1 viruses such as 1918 "Spanish" influenza viruses
- H7N9 viruses
All viruses used at the IRI are susceptible to current antiviral therapies such as Tamiflu and Relenza. In
addition, all laboratory researchers are immunized yearly with the seasonal influenza vaccine. When available,
researchers will also receive a H5N1 influenza vaccine.
In the event of an accidental exposure (i.e., a "known exposure") or the development of reportable influenzalike symptoms (listed below) without a known exposure, individuals must follow the procedures outlined below.
KNOWN EXPOSURE
Examples of Known Exposures
- Needle stick
- Animal bite
- Failure of personal protective equipment (PPE)
- A large spill of infectious agent outside of a biosafety cabinet
Response to Known Exposures
o In the event of a mucosal exposure, wash the affected area with running water for 15 minutes.
o In the event of a needle stick or an animal bite, spray the affected area with 70% EtOH, and
then wash with running water for 15 minutes.
o In the event of a large spill, follow the Large Biohazard Spill SOP (SOP #32). This type of a spill
is a potential exposure/release and is reportable to the CDC. Each situation will be evaluated by
Biosafety to determine the potential exposure risk to the individual and they will consult with the
infectious disease doctors to determine whether or not antiviral medications should be
prescribed or a quarantine period is necessary.
o Notify the on-call scientist by calling the emergency iPhone
researcher on the two-way radio.
or by contacting a
o The IRI on-call scientist will notify 1)
alternative (ARO).
and 2) the Responsible Official (RO) or
o The RO or ARO will contact the Infectious Disease (ID) consult team by calling the University of
Wisconsin (UW) hospital operator at __ The ID Consult team will determine what actions
are required. The RO or ARO will also promptly notify all appropriate local, State, and Federal
agencies.
o If medical attention is needed (e.g., stiches), the on-call scientist will bring the exposed
individual, who will be wearing an N-95 respirator without an exhalation valve, to the UW ER for
medical attention.

o If the exposure is of "high risk" defined as an exposure of the respira~ory tract or
mucous membranes with a recombinant virus containing one or more genes of the 1918
virus or a mammalian transmissible influenza virus of avian origin, the exposed
individual will be quarantined in an isolation room at the University of Wisconsin
Hospital. The on-call scientist will transport the exposed individual who will be wearing
an N-95 respirator without an exhalation valve to the University of Wiscon$in Hospital.
The individual will be quarantined until the Wisconsin Department of Public Health has
determined it is appropriate for the individual to return to their personal residence.
o If the exposure is of "high risk" defined as an exposure of the respiratory tract or
mucous membranes with a non-transmissible influenza virus of avian origin,,the exposed
individual will be quarantined to their personal residence provided they have started on
the treatment dose of Tamiflu (75mg twice a day) within 5 hours of the time of exposure.
o If the exposure is of "low to moderate risk" defined as an exposure not involving the
respiratory tract or mucous membranes with any BSL-3 influenza virus, the exposed
individual will be quarantined to their personal residence provided they have started on
the treatment dose of Tamiflu (75mg twice a day) within 5 hours of the time of exposure.
o Individuals will be prescribed antiviral medications by the ID consult team and will be
quarantined for 4-14 days after the exposure, as determined by state health officials. Individuals
will adhere to the guidelines in the University of Wisconsin "Quarantine and Isolation Policy."
o During the quarantine period, throat gargles, throat swabs, and nasal swabs will be collected
from the individual at the quarantine apartment twice a day (A.M. and P.M.) by trained UW staff
from the individual and diagnostic testing will be performed as described below. rm addition, the
quarantined individual will record their own bod tern erature (A.M. and P.M.) and email or
phone this information to the on-call scientist and ~;,~!~~~i°Y The health of the individual will
be monitored by medical professionals at the U unng vIsI ations. A daily summary of the·
diagnostic tests and temperatures will be emailed to all parties involved.
o If, during the home quarantine period, the individual develops influenza-like symptoms and/or
tests positive for influenza virus, the individual, who will be wearing an N-95 respirator without
an exhalation valve, will be transferred to the UW hospital for medical care in an isolation room.
o If the individual does not develop any influenza-like symptoms and/or repeatedly tests negative
for influenza virus, the individual will be released at the end of the quarantine period.
UNKNOWN EXPOSURE WITH REPORTABLE INFLUENZA-LIKE SYMPTOMS
If a researcher who has been working with the viruses previously listed, develops influenza symptoms
within 1 0 da s after their last BSL-3 entry, the researcher must immediately contact the on-call scientist and
Redacted by agreement
Reportable Symptoms:
Fever (temperature> 100° F)
Myalgia - sore muscles, body aches
Nonproductive cough - dry cough
Rhinitis - runny nose
Sore throat
Conjunctivitis - red, swollen eyelids
Fatigue (when clustered with other symptom(s))
Headache (when clustered with other symptom(s))
Response to Unknown Exposures with Reportable Symptoms:

o A throat gargle, throat swab, and nasal swab will be collected from the individual for diagnostic
testing (see below). Samples that are positive for 1918 (H1), H2, HS, or H7 viruses will be split
and sent to the Centers for Disease Control for independent verification of the results.
o Home quarantine of all household members is required until the diagnostic tests come back
negative. During this time period, individuals will adhere to the guidelines in the UW "Quarantine
and Isolation Policy."
o A positive diagnostic test for 1918 (H1 ), H2, HS, or H7 viruses will result in the transfer of the
individual, who will be wearing an N-9S respirator without an exhalation valve, to the UW
hospital for medical care.
o Individuals must disclose the agents (i.e., viruses) that they have been working with to the ID
consult team at the UW hospital.
o The names of all people in contact with the infected individual will be provided to University
Health Services within 48 hours.
o If a researcher tests positive for 1918 (H1), H2, HS, or H7, then all other household members
will be quarantined for 4-14 days, as determined by state health officials.
o During the quarantine period, throat gargles, throat swabs, and nasal swabs will be collected
from all household members twice a day (A.M. and P.M.) by trained UW staff. In addition,
quarantined individuals will record their temperatures twice a day (A.M. and P.M.) and email or
phone this information to the on-call scientist. The health of th~ individual will be monitored by
medical professionals at the UW during home visitations. A daily summary of the diagnostic
tests and temperatures will be emailed to all parties involved.
o If during the quarantine, household member(s) develop influenza-like symptoms and/or test
positive for influenza virus, the individual(s), who will be wearing an N-95 respirator without an
exhalation valve, will be transferred to the UW hospital for medical care in an isolation room.
o If household member(s) do not develop any influenza-like symptoms and/or test negative for
influenza virus, the individual(s) will be released at the end of the end of the quarantine period.
DIAGNOSTIC TESTING
o Individuals must give the required samples for diagnostic testing. These include
Throat gargles
Throat swabs
• Nasal swabs
o Samples will be collected by trained members of the swab team (IRI researchers or other UW
staff members).
o A rapid diagnosis Directigen AB kit for influenza virus and established RT-PCR protocols will be
conducted for H1, H2, H3, HS and H7 influenza viruses, as required, and will be completed
within 8 hours from the sample collection.
o Samples positive for 1918 (H1 ), H2, HS, or H7 will be split and sent to the Centers for Disease
Control for the independent verification of results.
CALL SYSTEM FOR REPORTING KNOWN EXPOSURES AND INFLUENZA-LIKE SYMPTOMS
The following people should be notified immediately after a known exposure occurs, or as soon as influenzalike symptoms develop:
o The IRI on-call scientist via the on-call iPhone
0 fedacted by agreement I
o The on-call scientist will notify the RO or ARO at the Office of Biological Safety
Contact Information:
Principal Investigator Yoshihiro Kawaoka
Responsible Official
Alternate Responsible Official
Alternate Responsible Official
Scientist
Scientist
Researcher
• Scientist
Revised: 12/2013 redacted by agreement

December 20, 2013
Jacqueline Corrigan-Curay, M.D., J.D.
Acting Director
Office of Biotechnology Activities
National Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda, MD 20892
Dear Dr. Corrigan-Curay:
-THE UNIVERSITY
WISCO_N_S1-N
MADISON
Thank you for the opportunity to respond to your letter dated December 16, 2013 regarding questions
NIH OBA has about a small spill onto the floor of one our ABSL-3+ laboratories. Specifically, corrective
action was requested in regards to exposed skin around the ankle of the researcher involved in the
incident.
In an effort to comply with the spirit of the guidelines and in response to your memo, the principle
investigator of the laboratory where the spill occurred has ordered high-top booties that will be worn
underneath the tyvek suit and inside of the dedicated clogs which will be covered with normal shoe
covers. Included with this memo are copies of the updated standard operating procedures for entry into
the principle investigator's laboratories incorporating the change with the PPE.
A highest priority at UW-Madison is to assure that we are in complete compliance with federal
regulations regarding select agent research. However, on occasion we have received mixed messages
from agencies regarding aspects of our program, and this is a case in point. This was the first instance
where we have been informed that our personal protective equipment (PPE) for RG3 influenza viruses
was inadequate. Our researchers wear P APRs, tyveks, shoe covers, dedicated garden clogs, shoe covers,
and two pairs of gloves. Our university's select agent program has been inspected IO separate times since
the creation of the Federal Select Agent Program (FSAP) with 8 of those inspections involving RG3
influenza viruses. During these inspections, FSAP Inspectors from the Centers for Disease Control (CDC)
and Animal and Plant Health Inspection Service (APHIS) enter into our laboratories wearing full PPE and
exit in the same manner as our researchers do on daily basis. Our most recent FSAP inspection was in
August 2013 and there was no mention of the PPE being inadequate. As required by the Federal Select
Agent Program we notified the CDC and APHIS of the spill within a few hours of the incident and the
report was reviewed by both agencies with APHIS handling the correspondence since the agent involved
falls under their purview. We received a memo dated November 20, 2013 from an APHIS compliance
manager, who reviewed our spill report that stated "At the time of incident the researcher was wearing
appropriate personal protective equipment." Since our phone conference call with NIH on December 16,
Graduate School
Bascom Hall Univenity of Wiscomin-1.fadison 500 Lincoln Drive lfadison, \\'7153706-1380
Dean's Office
60B-262-1044
Fax: 608/262-5134
Graduate Admissions & Academic
Sen-ices, Diversity Resources
608/26.2-2433, F.ar. 608/265-9505
Accounting
608/262-S83S
Fax: 606/262-5134
HUD1an Resources
608/262-5802
l¼x: 608/262-5235
Profusional Development
& Engagttn1ent
608/262-2433. F-ax: 608/26.2-5134

Jacqueline Corrigan-Curay, M.D., J.D.
December 20, 2013
Page2
2013 we have received a more recent memo from AP HIS dated December 17, 2013 stating after further
review, they are concerned about the bare skin.
We are requesting clarification from NIH Office of Biotechnology Activities (OBA) in regards to this
policy. Appendix G-II-C-5-a ( I) does not specifically mention the requirement of no exposed skin nor
does it discuss the type of PAPR a researcher working with RG3 influcn:r.a viruses should wear. As you
know, many PAPRs lack shrouds and leave the researcher's neck exposed. Does the no-bare-skin
requirement apply for all RG3 influenza viruses including wild type viruses or just mutant/reassortant
viruses? The reason we ask is we would like to be consistent across all RG3 influenza virus laboratories at
our institution. Further, we assume that many other institutions engaged in RG3 influenza research use
safeguards similar to ours. They also would benefit from clarification of exposed skin and PAPR
requirements.
Please contact me if you have any questions or concerns.
Sincerely,
Redacted by agreement
Associate Vice Chancellor for Research Policy
Attachments: Three SOPs for entry to facilities
Entry into BSL-3+ (
The normal hours of operation for the BSL-3 suites are from 6:00 a.m. to 10:00 p.m.
If you are oin to be in the BSL-3 suites after 8:00 p.m., you must notify the person on-call at
and noti edacted by agreement y email.
IRI SOP#S
Individuals working in the BSL-3+ suite must wear the following personal protective equipment (PPE): •
- a water resistant Tyvek suit
- disposable latex or nitrile exam gloves
- dedicated shoes covered by a pair of shoe covers (blue)
- a powered air purifying respirator (PAPR) must be worn when working with highly pathogenic avian
influenza (HPAI) viruses.
To ensure exposed .skin around the wrists and ankles is properly covered, researchers are required to wear
long cuffed gloves and/or protective sleeves and white Tyvex foot covers.
If you choose to wear an N100 respirator for work with Ebola~VP30 viruses or during periods when no virus
materials are being used in the suite, you must also wear:
- a hair cover
- safety glasses
PPE can be found in the clean gown room - along with posted signs listing the procedures for
entering the BSL-3+ suite.
Entry Procedure:
1) Fill-in the "BSL-3+ Sign-In/Out" sheet (Form# 13a).
2) Enter the clean gown room (-. turn on the In Use light, remove all street clothes, and change into
scrubs.
3) Put on the white Tyvek foot covers, Tyvek suit, dedicated laboratory shoes covered by shoe covers (blue),
tong cuffed gloves and/or protective sleeves and either a PAPR or an N100 with a hair cover and safety
glasses.
4) Walk through shower into the dirty gown room-·
5) If you are wearing a PAPR:
o Test the battery using the indicator strip on the back-side of the battery
o Perform an airflow test to verify that the HEPA filter is not clogged
• Insert the airflow indicator into the blower unit and turn the unit on
• The middle of the test ball must pass the "6 cfm" line
If the airflow test fails, select a different blower unit. Affix a label to the malfunctioning
blower unit and report the test failure to the tab supervisor
o Check to make sure the HEPA filter cover and hose is securely attached to the blower unit, and
the hose is securely attached to the hood. In addition, make sure there are no tears or
punctures in the hood.
6) Using the secure key access, enter the BSL-3 laboratory and turn off the In Use light.
Revised 12/17 /2013jRedacted by agreement

IRI SOP# 7
Entry into ABSL-3+ ( )
The normal hours of operation for the BSL-3 suites are from 6:00 a.m. to 10:00 p.m.
If you are going to be in the BSL-3 suites after 8:00 p.m., you must notify the person on-call at
and notifyfedacted by agreement !byemai I. •
Individuals working in the ABSL-3+ suite must wear the following personal protective equipment (PPE):
a water resistant Tyvek suit
disposable latex or nitrile exam gloves (two pairs of disposable gloves if working with animals)
dedicated shoes covered by a pair of shoe covers (blue)
a powered air purifying respirator (PAPR) must be worn when working with virus materials.
To ensure exposed skin around the wrists and ankles is properly covered, researchers are required to wear
long cuffed gloves and/or protective sleeves and white Tyvex foot covers.
If you choose to wear a N 100 respirator during periods when no virus materials are being used in the suite, you
must also wear:
a hair cover
safety glasses
If you are working with potential mammalian transmissible influenza viruses, you must also wear:
a second pair of disposable latex or nitrile exam gloves
protective sleeves
PPE can be found in the clean anterior room(-) of the ABSL-3+ suite along with posted signs listing
the procedures for entering the ABSL-3+ suite.
Entry Procedure:
1) Enter the men's or women's locker room (
2) Remove all street clothes in the locker room and change into scrubs, shoe covers over your feet, hall shoes,
and shoe covers over the hall shoes.
3) Bring a clean set of scrubs and a towel with you and then exit the locker rooms into the secure clean
corridor.
4) Fill-in the "BSL-3+ Sign-In/Out" sheet (Form# 13b).
5) Turn on the In Use light, enter into clean gown room ( ) using the
remove hall shoes.
6) Put on the white Tyvek foot covers, Tyvek suit, dedicated laboratory shoes covered by shoe covers (blue),
long cuffed gloves and/or protective sleeves. If you choose to wear a N100 respirator, also put on a hair cover
and safety glasses.
If you are working with potential mammalian transmissible influenza viruses, you must also wear a second pair
of disposable gloves and protective sleeves.
7) Walk through the shower.
8) If you are wearing a PAPR:
o Test the battery using the indicator strip on the back-side of the battery

o Perform an airflow test to verify that the HEPA filter is not clogged
Insert the airflow indicator into the blower unit and turn the unit on
The middle of the test ball must pass the "6 cfm" line
If the airflow test fails, select a different blower unit. Affix a label to the malfunctioning blower
unit and report the test failure to the lab supervisor
o Check to make sure the HEPA filter cover and hose is securely attached to the blower unit, and
the hose is securely attached to the hood. In addition, make sure there are no tears or
punctures in the hood.
9) Enter the BSL-3 laboratory through the dirty gown room(_), and turn off the In Use light.
Revised 12/17/2013jRedacted by agreement

Entry into ABSL-3Ag
The normal hours of operation for the BSL-3 suites are from 6:00 a.m. to 10:00 p.m.
If you are going to be in the BSL-3 suites after 8:00 p.m., you must notify the person on-call at
and notifylRedacted by agreement lby email.
IRI SOP#9
Individuals working in the ABSL-3Ag suites must wear the following personal protective equipment (PPE):
a water resistant Tyvek suit
two pairs of disposable latex or nitrile exam gloves
dedicated shoes covered by a pair of shoe covers (blue)
a powered air purifying respirator (PAPR) must be worn when working with virus materials
To ensure exposed skin around the wrists and ankles is properly covered, researchers are required to wear
long cuffed gloves and/or protective sleeves and white Tyvex foot covers.
lf you choose to wear an N100 respirator during periods when no virus materials are being used in the suite,
you must also wear:
a hair cover
safety glasses
If you are working with potential mammalian transmissible influenza viruses, you must also wear:
protective sleeves
PPE can be found in the clean gown room (- of the ABSL-3Ag suite along with posted signs listing
the procedures for entering the ABSL-3Ag suite.
1) Enter the men's or women's locker room (
2) Remove all street clothes in the locker room and change into scrubs, shoe covers over your feet, hall shoes,
and shoe covers over the hall shoes.
3) Bring a clean set of scrubs and a towel with you and then exit the locker rooms into the secure clean
corridor.
4) Fill-in the MBSL-3+ Sign-In/Out" sheet (Form# 13c).
5) Turn on the In Use light, enter into clean gown room (- using the
remove hall shoes.
6) Put on the white Tyvek foot covers, Tyvek suit and long cuffed gloves and/or protective sleeves. If you
choose to wear an N100 respirator, also put on a hair cover and the N100 respirator. •
If you are working with potential mammalian-transmissible influenza viruses, you must also wear protective
sleeves.
7) Leave the clean set of scrubs in a locker and take the towel with you. Enter the shower room through the
Presray door (SOP# 10). Hang the towel on a hook on the clean side of the shower.
8) Walk through the shower and into the dirty gown room (- through the Presray door.
9) If wearing a PAPR:
o Test the battery using the indicator strip on the back-side of the battery
o Perform an airflow test to verify that the HEPA filter is not clogged

Insert the airflow indicator into the blower unit and turn the unit on
The middle of the test ball must pass the "6 cfm" line
If the airflow test fails. select a different blow unit. Affix a label to the malfunctioning blower
unit and report the test failure to the lab supervisor
o Check to make sure the HEPA filter cover and hose is securely attached to the blower unit, and the
hose is securely attached to the hood. In addition, make sure there are no tears or punctures in the
hood.
10) Put on the dedicated laboratory shoes, shoe covers (blue) over the shoes, and a second pair of disposable
gloves. If wearing an N100, put on safety glasses.
11) Enter into ABSL-3Ag hallway and turn off the In Use light
12) Follow the posted signs on the inside of the laboratory doors that outline the procedures for changing shoe
covers, outer gloves and protective sleeves every time you leave each laboratory.
Revised 12/17/2013 redacted by agreement

, .......... (,-'!/-loo.&:J) National Institutes of Health
December 17, 2013
redacted by agreement I
Vice Chancellor for Research and Dean of the Graduate School
University of Wisconsin
3 3 3 Bascom Hall
500 Lincoln Drive
Madison, WI 53706-1380
Dear redacted by agreement
U.S. Public Health Service
Bethesda, Maryland 20892
Office of Biotechnology Activities
National Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda, MO 20892-7985
(301) 496-9838 (Phone)
(301) 496-9839 (Fax)
http://oba.od.nih.gov/
We are writing in regard to two incidents involving recombinant research with highly pathogenic
avian influenza HPAI) H5Nl that have occurred recently in the ABSL3+ laboratory of Dr.
Redacted by agreement fter reviewing the details of these two incidents, NIH has significant
concerns relating to the University of Wisconsin's apparent lack of a dedicated quarantine
facility other.than the researcher's home. We also have concerns relating to the biosafety
practices associated with these incidents. Our concerns are detailed below.
Lack ofa dedicated quarantine facility
In the needlestick incident that occurred on November 16, 2013, a decision was made to home
quarantine the individual because the route of exposure (needlestick) was not expected to place
the researcher at high risk for infection and this influenza strain, which contained the HA gene
from H5Nl, was determined not to be a mammalian-transmissible strain. However, in
conversations with the Universit of Wisconsin Alternate Responsible Official, 1=Re--d-'-act.,...e..,.d b,...y-ag-re-e-me-nt,.......,
edacted by egarding this inciden edacted by nformed us that all researchers exposed to H5Nl areement • areement
. would be quarantined at home, regar ess o the risk of infection or whether the strain was
mammalian-transmissible or not.
In a subsequent phone conversation with the University of Wisconsin Senior Associate Dean for
Research Redacted by agreement the lie for home isolation for all incidents was
reiterated to us. We were told by edacted by agreement that the decision was based upon
consultation with University of Wisconsin infectious disease experts and the state health
department. We were also informed that the use of a hospital room for quarantine was rejected
due to the stress it would place on the laboratory worker.

redacted by agreement
December 16, 2013
Page2
The University of Wisconsin's policy on home quarantine communicated to us bylRedacted byagreementl
F edacted by agreementjis not in keeping with what was communicated to us in fedacted by agreement I
application to the Department of Health and Human Services to perform research with
mammalian transmissible strains ofHPAI HSNl. In a May 6, 2013, plan provided to NIH, Dr.
• ndicated that he had access to a "designated quarantine apartment" in which
researc ers could be laced for 10-14 days in the event of an accidental exposure (Attachment
A). Redacted by agreement ave indicated to OBA that there was a miscommunication
between the PI and the University of Wisconsin administration regarding the availability and
appropriateness of such a quarantine apartment.
The University of Wisconsin's policy on home quarantine is inconsistent with the requirements
for this research under the NIH Guidelines for Research Involving Recombinant or Synthetic
Nucleic Acid Molecules (NIH Guidelines), and under the terms agreed to by the University as a
condition of funding this project. The University of Wisconsin must find a dedicated facility
outside of the individual's permanent residence (1} in which an individual exposed to
mammalian-transmissible HPAI HSNl can be safely isolated for up to 10 days, and (2) that
can be decontaminated easily after the individual's departure. An isolation room in a
hospital would also be appropriate. An individual's permanent residence is not appropriate when
the risk of infection is high. For high risk exposures, it is critical to isolate the individual in a
structure that does not have shared air exchange and can be quickly and efficiently
decontaminated in the case of infection. In addition, if this structure is outside of a health care
facility, there needs to be a plan in place regarding how this researcher could be safely
transported to an isolation room in a health care facility, should he or she develop clinical
symptoms, without the risk of exposure to other individuals.
Concerns relating to hiosafetv practices
In addition to the quarantine issue, NIH has significant concerns regarding the biosafety practices
associated with both of the recent incidents.
The November 16, 2013, needlestick incident occurred when the researcher used a needle to
collect tissue culture supernatant in violation of the University of Wisconsin's own policies,
which only permits needles to be used in the ABSL3+ laboratory to anesthetize research animals,
draw blood from research animals, or inoculate eggs. It was unclear from the University's
response why this individual was using a needle for this type of procedure.
The University of Wisconsin report regarding the November 9, 2013, HPAI HSNl spill
described the researcher as having two to three inches of exposed skin between where his tyvek
suit ended and his shoe covers began. While it was reported that none of the spilled material
landed on the researcher's bare skin, we made it clear in our letter (Attachment A) and in a
phone conversation withfedacted by agreement I that having bare skin in the ABSL3+
laboratory was unacceptable under the containment requirements for this research specified in
the NIH Guidelines. During that phone conversation, redacted by agreement I stated that the
ABSL3+ laboratory had recently undergone a Select Agent inspection and the report from that
inspection did not specifically mention a prohibition against working in the ABSL3+ laboratory

redacted by agreement
December 16, 2013
Page3
with bare skin. We have discussed the issue of bare skin in the ABSL3+ laboratory with the
United States Department of Agriculture (USDA) Select Agent Program, and they are in
agreement that bare skin is unacceptable at this level of containment.
Attachments B and Attachment C to this letter contain the NIH response to both HSN I incidents.
These letters contain requests for action regarding the quarantine situation and our biosafety
concerns. We would appreciate any assistance you can provide to ensure that these requests are
answered by December 23, 2013.
Finally, if your response is not received by this date or if does not fully address the issues we
have described regarding a dedicated quarantine facility and inappropriate biosafety practices, as
required by the terms and conditions of rant award, NIH will institute enforcement action(s) for
the NIH gran Redacted by agreement
!Redacted by agreement I Principal Investigator. Such actions could include disaUowance of
costs, suspension, or termination of the grant award.
If you have any questions, please feel free to contact us._ ---- ..
0 at:) --, c;;,,,r.7/--~ -z:_/ {,;~! 1? v. = - ,,-- 7#" /1/ /
Amy f Patterson, M.D. Sally Rockey, Ph.D.
Associate Director for Science Policy Deputy Director for Extramural Research
National Institutes of Health National Institutes of Health
cc: "-edacted by agreement
Capt. Robbin Weyant, Ph.D., Director, Division of Select Agents and Toxins, CDC
Freeda E. Isaac, D.V.M., Director, Agriculture Select Agent Program, USDA APHIS
_ Jacqueline Corrigan-Curay, J.D., M.D., Acting Director, Office of Biotechnology Activities, NIH

ATTACHEMENT A
MAY 6, 2013, UNIVERSITY OF WISCONSIN INFLUENZA RESEARCH INSTITUTE
EXPOSURE PLAN (redacted)

IRI SOP#48
Exposure Plan - Influenza Viruses
At the Influenza Research Institute (IRI) the following influenza viruses are used in our BSL-3+ and BSL-3
Agriculture (Ag) laboratories:
All viruses used at the IRI are susceptible to current antiviral therapies such as Tamiflu and Relenza. In
addition. all laboratory researchers are immunized yearly with the seasonal influenza vaccine. When available,
researchers will also receive a HSN1 influenza vaccine.
In the event of an accidental exposure (i.e., a "known exposure") or the development of reportable influenzalike symptoms (listed below) without a known exposure, individuals must follow the procedures outlined below.
KNOWN EXPOSURE
Examples of Known Exposures
- Needle stick
- Ani ma I bite
- Failure of personal protective equipment (PPE)
- A large spill of infectious agent outside of a biosafety cabinet
Response to Known Exposures
o tn the event of a mucosa! exposure, wash the affected area with running water for 15 minutes.
o In the event of a needle stick or an animal bite, spray the affected area with 70% EtOH, and
then wash with running water for 15 minutes.
o In the event of a large spill, follow the Large Biohazard Spill SOP (SOP #32).
o Notify the on-call scientist by calling the on-call iPhone
researcher on the two-way radio.
or by contacting a
o The IRI on-call scientist will notify 1 ~~-ed-act_e_d_bY __ ~land 2) the Responsible Official (RO) or ~qreement .
alternative (ARO).
o The RO or ARO will contact the Infectious Disease (ID) consult team by calling the University of
Wisconsin (UW) hospital operator at -- The ID Consult team will determine what actions
are required. The RO or ARO will also promptly notify all appropriate local, State, and Federal
agencies.
o If medical attention is needed (e.g., stiches), the on-call scientist will bring the exposed
individual, who will be wearing an N-95 respirator without an exhalation valve, to the UW ER for
medical attention.
a If no medical attention is needed, the on-call scientist will bring the exposed individual, who will
be wearing an N-95 respirator without an exhalation valve, to the designated "quarantine
apartment."

o Individuals will be prescribed antiviral medications by the ID consult team and will be
quarantined for 10-14 days after the exposure, as determined by state health officials.
Individuals will adhere to the guidelines in the University of Wisconsin "Quarantine and Isolation
Policy."
o During the quarantine period, throat gargles, throat swabs, and nasal swabs will be collected
from the individual at the quarantine apartment twice a day (A.M. and P.M.) by trained UW staff
from the individual at the Quarantine Apartment and diagnostic testing will be performed as
described below. ln addition, the quarantined individual will record their own body temperature
(A.M. and P.M.) and email or phone this information to the on-call scientist and!Redacteooyagreement
The health of the individual will be monitored by medical professionals at the UW during
visitations at the quarantine apartment. A daily summary of the diagnostic tests and
temperatures will be emailed to all parties involved.
o If, during the quarantine period, the individual develops influenza-like symptoms and/or tests
positive for influenza virus, the individual, who will be wearing an N-95 respirator without an
exhalation valve, will be transferred by emergency medical services (EMS) to the UW hospital
for medical care in an isolation room.
o If the individual does not develop any influenza-like symptoms and/or repeatedly tests negative
for influenza virus, the individual will be released at the end of the quarantine period.
UNKOWN EXPOSURE WITH REPORTABE INFLUENZA-LIKE SYMPTOMS
If a researcher who has been working with the viruses previously listed, develops influenza symptoms
within 10 da s after their last BSL-3 entry, the researcher must immediately contact the on-call scientist and
edacted by agreement
Reportable Symptoms:
Fever (temperature> 100° F)
Myalgia
Nonproductive cough
Rhinitis
Sore throat
Conjunctivitis
Fatigue (when clustered with other symptom(s))
Headache (when clustered with other symptom(s))
Response to Unknown Exposures with Reportable Symptoms:
o A throat gargle, throat swab, and nasal swab will be collected from the individual for diagnostic
testing (see below). Samples that are positive for 1918 (H1 ), H2, H5, or H7 viruses will be split
and sent to the Centers for Disease Control for independent verification of the results.
o Home quarantine of all household members is required until a minimum of two diagnostic tests
from different samples come back negative. During this time period, individuals will adhere to
the guidelines in the UW "Quarantine and Isolation Policy."
o A positive diagnostic test for 1918 (H1), H2, H5, or H7 viruses will result in the transfer of the
individual, who will be wearing an N-95 respirator without an exhalation valve, by EMS to the
UW hospital for medical care.
o Individuals must disclose the agents (i.e., viruses) that they have been working with to the ID
consult team at the UW hospital.
o The names of all people in contact with the infected individual will be provided to University

Health Services within 48 hours.
o If a researcher tests positive for 1918 ( H 1), H2, H5, or H7, then all other household members
will be quarantined for 10-14 days, as determined by state health officials.
o During the quarantine period, throat gargles, throat swabs, and nasal swabs will be collected
from all household members twice a day (A.M. and P.M.) by trained UW staff. In addition,
quarantined individuals will record their temperatures twice a day (A.M. and P.M.) and email or
phone this information to the on-call scientist. The health of the individual will be monitored by
medical professionals at the UW during home visitations. A daily summary of the diagnostic
tests and temperatures will be emailed to all parties involved.
o If during the quarantine, household member(s) develop influenza-like symptoms and/or test
positive for influenza virus, the individual(s), who will be wearing an N-95 respirator without an
exhalation valve, will be transferred by EMS to the UW hospital for medical care in an isolation
room.
o If household member(s) do not develop any influenza-like symptoms and/or test negative for
influenza virus, the individual(s) will be released at the end of the end of the quarantine period.
DIAGNOSTIC TESTING
o Individuals must give the required samples for diagnostic testing. These include
Throat gargles
• Throat swabs
• Nasal swabs
o Samples will be collected by trained UW laboratory staff members.
o A rapid diagnosis Directigen AB kit for influenza virus and established RT-PCR protocols will be
conducted for H1, H2, H3, H5 and H7 influenza viruses, as required, and will be completed
within 8 hours from the sample collection.
o Samples positive for 1918 (H1), H2, H5. or H7 will be split and sent to the Centers for Disease
Control for the independent verification of results.
CALL SYSTEM FOR REPORTING KNOWN EXPOSURES AND INFLUENZA-LIKE SYMPTOMS
The following people should be notified immediately after a known exposure occurs, or as soon as influenzalike symptoms develop:
o The \RI on-call scientist via the on-call iPhone
0 !Redacted by agreement I
o The on-call scientist will notify the RO or ARO at the Office of Biological Safety
Contact Information:

---··--- - - --- -- - -- - . - - - - - -· - - -------- -~------
Revised: 05/06/2013IRedacted by agreement

ATTACHEMENT B
NIH OBA RES~ONSE TO UNIVERSITY OF WISCONSIN'S NOVEMB,ER 9, 2013,
HSNl SPILL

... ~., ( •> National Institutes of Health
December 16, 2013
f edacted by agreement
Associate Vice Chancellor for Research Policy
University of Wisconsin
327 Bascom Hall 3
500 Lincoln Drive
Madison, Wisconsin 53 706
Dear redacted by agreement
U.S. Public Health Service
Bethesda, Maryland 20892
Office of Biotechnology Activilles
Natlonal Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda, MD 20892-7985
(301) 496-9838 (Phone)
(301) 496-9839 (Fax)
http://oba.od.nlh.gov/
We are following up on our series of teleconferences regarding your November 15, 2013,
report to the National Institutes of Health (NIH) Office of Biotechnology Activities (OBA)
describing a November 9, 2013, incident in which a University of Wisconsin researcher spilled
approximately 8 mL ofH5Nl'-containing media onto the floor of the ABSL3+ laboratory.
According to your report, the spill occurred shortly after the researcher collected H5Nl culture
. supernatant samples into three 6-well tissue culture plates. The researcher stacked the three
plates on top of each other and transported the plates from the biosafety cabinet to the tissue
culture incubator. After opening the door of the incubator, the researcher dropped one of the 6-
well plates onto the laboratory floor.
Immediately following the spill, the researcher closed the door to the incubator and returned the
well plates that were not dropped to the biosafety cabinet. The researcher then picked up the
dropped plate and immersed it in a chemical disinfectant solution within the biosafety cabinet.
He then saturated his outer gloves with 70-percent ethanol, disposed of them in the biohazrdous
waste bin and donned a new pair of outer gloves. At this time, the researcher noticed some of
the spilled material had landed on his Tyvek suit just below the knee. In response, he applied 70- •
percent ethanol to both the arms and legs of the suit, his shoe covers, and the uncovered skin
between the bottom of the suit and his shoes. Next, the researcher cleaned the spill by covering
the affected area with paper towels soaked in chemical disinfectant. After allowing 20 minutes
for the towels to soak up the spilled material, the researcher mopped the floor using a viral
disinfectant. The biosafety cabinet and the tissue culture incubator were then decontaminated
using 70-percent ethanol. All cleanup materials were placed in a biohazardous waste bag for
autocJaving. When the spill was fully cleaned up, the researcher phoned the on-call scientist to
report th<; incident, removed his personal protective equipment, and reported to the building
conference room to wait for further instructions.

redacted by agreement
December 16, 2013
Page2
When the on-call scientist received the notification of the spill, he phoned the Alternate
Responsible Official (ARO) and left a message describing the situation. The ARO returned the
call, and was brief ed. The ARO then called the researcher involved in the incident to obtain
additional information. She infonned the researcher to remain in the conference room while she
consulted with the University of Wisconsin infectious disease physician. After considering the
facts of the incident, the infectious disease physician determined that treatment with antivirals
would not be necessary. This determination was based on the fact that the appropriate
disinfection procedures had been used, and the risk of infection from any skin exposure would be
low. The ARO nonetheless insisted that the researcher be given a prescription for Tami flu as a
precautionary measure. The infectious disease physician agreed, and a prescription was written
for the researcher. At that time, the ARO called the researcher and informed him that he was
cleared to leave the building, and quarantine would not be necessary. The researcher was
instructed to self-monitor for any change in body temperature and report any feelings of illness
he experienced. The researcher began the Tamiflu course of treatment on the afternoon of •
November 9. To date, no symptoms of illness have been reported by the researcher.
While the actions taken by the University of Wisconsin in response to this incident appear
appropriate, we do not consider it acceptable biosafety practice to have uncovered skin in an
ABSL3+ laboratory. It was unclear from your original report whether any of the spilled media
splashed onto the bare ankles of the researcher. In subsequent communications, the University
of Wisconsin informed us that the researcher did not spill any of the material on his ankles; the
spill was limited to the researcher's Tyvek suit and the laboratory floor. That said, to be
compliant with Appendix G-11-C-5-a-(l) of the NIH Guidelines, researchers must be fully
covered with no exposed skin when perfonning research with RG3 influenza strains, including
HPAI HSNl. We have discussed the issue of bare skin in the ABSL3+ laboratory with the
United States Department of Agriculture (USDA) Select Agent Program, and they are in
agreement that bare skin is unacceptable at this level of containment. The University must take
immediate action to ensure that, in the future, no workers in this or any other high containment
laboratories have exposed skin. Please provide us with a corrective action that details how
the University of Wisconsin will address this deficiency no later than December 23, 2013.
Please contact OBA staff by email at oba@od.nih.gov or by telephone at (301) 496-9838 if you
have any questions.
Sincerely,
~l; C 1·
cque me om
cting Director
ffice of Biotechnology Act ities
I
I
I
)
I
I
I
I
I

redacted by agreement
December 16, 2013
Page 3
cc: Redacted by agreement
Capt. Robbin Weyant, Ph.D., Director, Division of Select Agents and Toxins, CDC
Freeda E. Isaac, D.V.M., Director, Agriculture Select Agent Program, USDA APHIS
Teresa Hauguel, Program Officer, NIAID, Nlli
Amy P. Patterson, M.D., Associate Director for Science Policy, NIH
Allan C. Shipp, Director of Outreach, Office of Biotechnology Activities, NIH
Ryan Bayha, Senior Analyst for Science Policy Outreach, Office of Biotechnology Activities, NIH
Kathryn Harris, Ph.D., RBP, Senior Outreach and Education Specialist (contractor),
Office of Biotechnology Activities, NIH

ATTACHEMENT C
NIH OBA RESPONSE TO UNIVERSITY OF WISCONSIN'S NOVEMBER 16, 2013,
HSN! NEEDLESTICK

,,..pflQ ..
{_J Ill)) National Institutes of Health
December 16, 2013
!Redacted by agreement
Associate Vice Chancellor for Research Policy
University of Wisconsin
327 Bascom Hall 3
500 Lincoln Drive
Madison, Wisconsin 53706
DearlRedacted by agreement
U.S. Public Health Service
Bethesda, Maryland 20892
Office of Blotechnology Activities
National Institutes of Health
6705 Rockledge Drive
Suite 750, MSC 7985
Bethesda, MD 20892-7985
{301) 496-9838 (Phone)
(301) 496-9839 (Fax)
http://oba.od.nlh.gov/
We are following up on our series of teleconferences regarding your November 17, 2013, and
November 19, 2013, report to the National Institutes of Health (NIH) Office of Biotechnology
Activities (OBA) describing a November 16, 2013, incident in which a University of Wisconsin
researcher stuck himself with a syringe containing a recombinant fonn of HSN 1. The virus
contained the HA gene from H5Nl while the remaining genes came from HlNl A/California
(2009 HlNl) strain.
The needlestick occurred in the ABSL3+ laboratory while the researcher was attempting to
collect tissue culture supernatant. Immediately following the incident, the researcher sprayed the
wound site with disinfectant and washed the site of the wound for fifteen minutes. The
researcher contacted the laboratory manager and reported the exposure. The laboratory manager
then infonned the Alternate Responsible Official (ARO) of the incident and requested that the
University of Wisconsin infectious disease physician be contacted for a consultation. The
laboratory manager called the researcher back and instructed him to put on a new pair of gloves,
clean up his work area, follow the shower-out procedures, and report to the building conference
room to await further instructions.
After the ARO briefed the infectious disease physician on the exposure, the University
determined that the researcher should be placed under quarantine at his home. The laboratory
manager phoned the researcher's family members and informed them of the situation. The
researcher's family was subsequently escorted to a hotel room for the duration of the researcher's
quarantine. The researcher was provided with a prescription of Tami flu and was then driven
home by the laboratory manager. During the transport from the laboratory to the researcher's
house, the researcher wore a glove on the hand that had the puncture wound as well as an N-95
respirator. The following morning (November 17), nasal and throat swab testing began. To date,
the researcher has not shown any signs or symptoms of illness.

!Redacted by agreement
December 16, 2013
Page 2
This event raises several questions regarding the University of Wisconsin's policies and
procedures. First, we learned in subsequent conversations with the University that the use of a
needle for the collection procedure in question is a violation of the University of Wisconsin's
own policies, which only permit needles to be used in the ABSL3+ laboratory to anesthetize
research animals, draw blood from research animals, or inoculate eggs. It is unclear from your
report why the researcher was using a syringe to coJJect a supernatant sample, when the use of
sharps for that activity was prohibited. Your report indicated that the researcher would be
retrained and the standard operating procedure for sharps use in the ABSL3+ will be rewritten to
articulate more clearly the allowed uses of sharps. Please indicate when this training occurred
and provide OBA copies of the training materials that were used, as well as your policy
describing the use of needles in the ABSL3+ laboratory.
In fi 11 . "th d edacted by agreement d" · al h Ith
o ow-up conversat10ns w1 you an r---,-..,......,r--....,...-~ egar mg your occupahon ea
plans, you state that all exposures, inclu mg ns exposures, would follow the same protocol,
i.e. home isolation after removing the family from the house. Your decision was based upon
consultation with your infectious disease experts and the state health department. You had
rejected using a hospital room for quarantine beca ft ss on the laboratory worker.
h. 1 · · h • d . Redacted by agreement ) ' • erfi T 1s po icy 1s not w at was commumcate to us 1 +TT"TT"r-n~.,,,,.,,~,,.._ pp 1cat10n to p arm
research with mammalian transmissible strains of at was provided to the D artment of
Health and Human Services. In a May 6, 2013, plan provided to NIH ;r~!~~~t
that he had access to a "designated quarantine apartment" in which researc ers cou be placed
for 10-14 days in the event of an accidental exposure. Since then, the University of Wisconsin's
Associate Dean for Research and ARO have indicated to OBA that there was a
miscommunication between the PI and the University administration regarding the availability
and appropriateness of such a quarantine apartment.
The University must f'md a dedicated facility outside of the individual's permanent
residence (1) in which an individual can be safely isolated for up to 10 days, and (2) that
can be decontaminated easily after the individual's departure. An isolation room in a
hospital would be appropriate. An individual's permanent residence is not appropriate due to the
fact that many residences are in buildings with high occupancy that share air exchange and other
infrastructure. Please provide revised SOPs that reflect an appropriate quarantine
arrangement. No research with mammalian transmissible H5Nl stains may be carried out
until this plan is operationalized.
Please provide all requested materials by December 23, 2013. You may contact OBA staffby
email at oba@od.nih.gov or by telephone at (30 l) 496-9838 if you have any questions.

!Redacted by agreement
December 16, 2013
Page 3
cc: edacted by agreement
Capt. Robbin Weyant, P .D., Director, D1vis1on o Se ect Agents an Toxms, C
Freeda E. Isaac, D.V.M., Director, Agriculture Select Agent Program, USDA APHIS
Amy P. Patterson, M.D., Associate Director for Science Policy, NIH
Allan C. Shipp, Director of Outreach, Office of Biotechnology Activities, NIH
Ryan Bayha, Senior Analyst for Science Policy Outreach, Office of Biotechnology Activities, NIH
Kathryn Hanis, Ph.D., RBP, Senior Outreach and Education Specialist (contractor),
Office of Biotechnology Activities, NIH

(.::J-. IIIJD} National Institutes of Heallh
December 16, 2013
redacted by agreement I
Associate Vice Chancellor for Research Policy
University of Wisconsin
327 Bascom Hall 3
500 Lincoln Drive
Madison. Wisconsin 53706
Dear f edacted by agreement I
U.S. Publlc Health Service
Bethesda, Mary1and 20892
Office of Biotechnology Activities
National lnsliltltes of Health
6705 Rockhtdge Drive
Suite 750, MSC 7985
Bethesda, MO 20892-7985
(301) 496-9838 (Phone)
(301) 496-9B39 ( Fax)
http://oba.od.nlh.gov/
We are following up on our series of teleconferences regarding your November I 7, 2013, and
November 19, 2013, report to the National Institutes of Health (NIH) Office of Biotechnology
Activities (OBA) describing a November 16, 2013, incident in which a University of Wisconsin
researcher stuck himself with a syringe containing a recombinant fonn of H5Nl. The virus
contained the HA gene from H5N1 while the remaining genes came from HlNl A/California
(2009 HlNl) strain.
The needlestick occurred in the ABSL3+ laboratory while the researcher was attempting to
collect tissue culture supernatant. Immediately following the incident, the researcher sprayed the
wound site with disinfectant and washed the site of the wound for fifteen minutes. The
researcher contacted the laboratory manager and reported the exposure. The laboratory manager
then infonned the Alternate Responsible Official (ARO) of the incident and requested that the
University of Wisconsin infectious disease physician be contacted for a consultation. The
laboratory manager called the researcher back and instructed him to put on a new pair of gloves,
clean up his work area, follow the shower-out procedures, and report to the building conference
room to await further instructions.
After the ARO briefed the infectious disease physician on the exposure, the University
determined that the researcher should be placed under quarantine at his home. The laboratory
manager phoned the researcher's family members and informed them of the situation. The
researcher's family was subsequently escorted to a hotel room for the duration of the researcher's
quarantine. The researcher was provided with a prescription of Tami flu and was then driven
home by the laboratory manager. During the transport from the laboratory to the researcher's
house, the researcher wore a glove on the hand that had the puncture wound as well as an N-95
respirator. The following morning (November 1 7), nasal and throat swab testing began. To date,
the researcher has not shown any signs or symptoms of illness.

redacted by agreement
December 16, 2013
Page2
This event raises several questions regarding the University of Wisconsin's policies and
procedures. First, we learned in subsequent conversations with the University that the use of a
needle for the col1ection procedure in question is a violation of the University of Wisconsin's
own policies, which only permit needles to be used in the ABSL3+ laboratory to anesthetize
research animals, draw blood from research animals, or inoculate eggs. It is unclear from your
report why the researcher was using a syringe to collect a supernatant sample, when the use of
sharps for that activity was prohibited. Your report indicated that the researcher would be
retrained and the standard operating procedure for sharps use in the ABSL3+ will be rewritten to
articulate more clearly the allowed uses of sharps. Please indicate when this training occurred
and provide OBA copies of the training materials that were used, as well as yottr policy
describing the use of needles in the ABSL3+ laboratory.
redacted by agreement I . In follow-up conversations with you and.__ ____ ___.regarding your occupational health
plans, you state that all exposures, including high risk exposures, would follow the same protocol,
i.e. home isolation after removing the family from the house. Your decision was based upon
consultation with your infectious disease experts and the state health department. You had
rejected using a hospital room for quarantine because of the stress on the laboratory worker.
This policy is not what was communicated to us in Redacted by agreemen pplication to perform
research with mammalian transmissible strains of at was provid t the D artment of
Health and Human Services. In a May 6, 2013, plan provided to NIH, R;r~!~~~ty ndicated
that he had access to a "designated quarantine apartment" in which researc ers cou be placed
for 10-14 days in the event of an accidental exposure. Since then, the University of Wisconsin's
Associate Dean for Research and ARO have indicated to OBA that there was a
miscommunication between the PI and the University administration regarding the availability
and appropriateness of such a quarantine apartment.
The University must f'md a dedicated facility outside of the individual's permanent
residence (1) in which an individual can be safely isolated for up to 10 days, and (2) that
can be decontaminated easily after the individual's departure. An isolation room in a
hospital would be appropriate. An individual's permanent residence is not appropriate due to the
fact that many residences are in buildings with high occupancy that share air exchange and other
infrastructure. Please provide revised SOPs that reflect an appropriate quarantine
arrangement. No research with mammalian transmissible H5Nl stains may be carried out
until this plan is operationalized.
Please provide all requested materials by December 23, 2013. You may contact OBA staff by
email at oba@od.nih.gov or by telephone at (301) 496-9838 if you have any questions.
turn~ (\
uray,M~p.
g 1rec or J
ice of Biotechnology Activities
V

!Redacted by agreement
December 16, 2013
Page 3
cc: Redacted by agreement
Capt. Ro m Weyant, P .D., 1rector, D1v1S1on of Se ect Agents and Toxins, CDC
Freeda E. Isaac, D.V.M., Director, Agriculture Select Agent Program, USDA APHIS
Arny P. Patterson, M.D., Associate Director for Science Policy, NIH
Allan C. Shipp, Director of Outreach, Office of Biotechnology Activities, NIH
Ryan Bayha, Senior Analyst for Science Policy Outreach, Office of Biotechnology Activities, Nill
Kathryn Harris, Ph.D., RBP, Senior Outreach and Education Specialist (contractor},
Office of Biotechnology Activities, NIH

Template for Reporting Incidents Involving Recombinant DNA to the NIH
Office of Biotechnology Activities (OBA)
The NIH Guidelines for Research Involvin~ Recombinant DNA Molecules (NIH Guidelines) states that" .. any
significant problems, violations of the NII/ Guidelines, or any significant research-related accidents and
illnesses" must be reported to NIH OBA within 30 days. Certain types of incidents must be reported on a more
expedited basis. Spills or accident~ in BSL-2 laboratorics resulting in an overt exposure must be immediately
reported to NIH OBA. Spills or accidents occurring in high containment (BSL-3 or BSL--4) laboratories
resulting in an overt or potential exposure must be immediately reported to NIH OBA_
This template is intended to facilitate the reporting of incidents that occur during the conduct of research
subject to the NIH Guidelines. Use of this template is not required and other formats may be acceptable.
A separate template for reporting Human Gene Transfer Adverse Events is available at:
http://www4.od.nih.gov/oba1rac/adverse __ event_ template.doc
Please note that submitting this completed template to the NIii OBA does NOT fulfill the reporting
requirements of other agencies. You should verify with the other parties to whom you must report whether the
use ofthis template is acceptable.
Completed reports may be sent via U.S. mail, courier service, e-mail, or facsimile to;
Attention: Incident Reports
NIH Office of Biotechnology Activities
6705 Rockledge Drive, Suite 750
Bethesda, Maryland 20892-7985
(For all non-USPS deliveries use Zip Code 20817)
Telephone 301-496-9838
FAX 301-496-9839
E-mail: oba@od.nih.gov
NIH OBA Incident Re(!orting Tem(!late
Does this incident involve research X Yes
subject to the MH Guidelines? If no, this incident docs not have to be rel"lClrted to OBA
Institution name: University of Wisconsin - Madison
Date of report: 11/19/13
Reporter name and position: Jim Turk Biological Safety Officer
Reporter telephone: (608)263-9013
Reporter email: jturk@fpm.wisc.edu
Date of Incident: 11116/13
Name of principal investigator: Yoshihiro Kawaoka
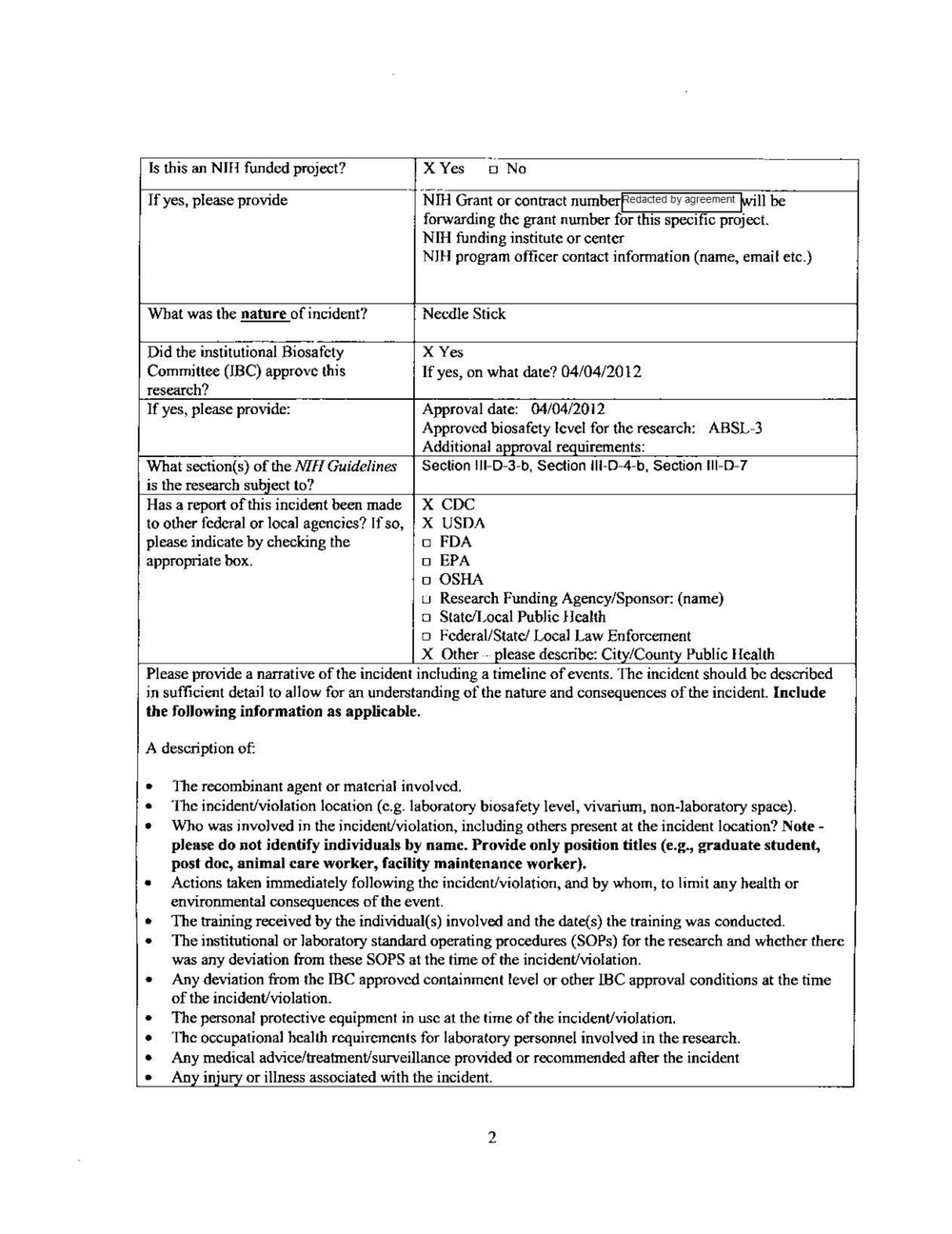
~--------------~-------------------- ------~ Is this an NIH funded project? X Yes No
If yes, please provide
What was the nature of incident?
Did the institutional Biosafcty
Committee (IBC) approve this
research?
If yes, please provide:
What section(s) of the NIH Guidelines
is the research su~ject to?
Has a report of this incident been made
to other federal or local agencies? If so,
please indicate by checking the
appropriate box.
-Nill Grant or contract numberfedacted by agreement t,vill be
forwarding the grant number for this specific project.
NIH funding institute or center
NJH program officer contact information (name, email etc.)
Needle Stick
XYes
If yes, on what date? 04/04/2012
Approval date: 04/04/2012
Approved biosafcty level for the research: ABSL-3
Additional aonroval re(]uirements:
Seclion 111-D-3-b, Section 111-D-4-b, Section 111-D-7
X CDC
X USDA
o FDA
EPA
OSHA
u Research Funding Agency/Sponsor: (name)
State/Local Public J lcalth
Federal/State/ Local Law Enforcement
X Other - please describe: City/County Public I lealth
Please provide a narrative of the incident including a timelinc of events. The incident should be described
in sufficient detail to allow for an understanding of the nature and consequences of the incident. Include
the following information as applicable.
A description of:
• The recombinant agent or material involved.
• ·inc incident/violation location (e.g. laboratory biosafety level, vivarium, non-laboratory space).
• Who was involved in the incident/violation, including others present at the incident location? Note -
please do not identify individuals by name. Prnvide only position titles (e.g., graduate student,
post doc, animal care worker, facility maintenance worker).
• Actions taken immediately following the incident/violation, and by whom, to limit any health or
environmental consequences of the event.
• The training received by the individual(s) involved and the date(s) the training was conducted.
• The institutional or laboratory standard operating procedures (SOPs) for the research and whether there
was any deviation from these SOPS at the time of the incident/violation.
• Any deviation from the IBC approved containment level or other IBC approval conditions at the time
of the incident/violation.
• The personal protective equipment in use at the time of the incident/violation.
• '!be occupational health requirements for laboratory personnel involved in the research.
• Any medical advice/treatment/surveillance provided or recommended after the incident
• Any injury or illness associated with the incident.
2

• Medical surveillance results (if not available at the time of initial report please indicate when results
will be available).
• Equipment failures.
DESCRIPTION OF INDICENT: (use additional space as necessary)
Needle Stick - November 16, 2013
~ 18:20-18:30 -The researcher was working in the ABSL3+ laboratory and accidentally punctured
skin with an 18 gauge needle with a reassortant virus on it, containing the HA from
ANietnam/UT36250l/2010 (H5Nl) with the Nl98H mutation in the receptor binding site, and the
rest of the genes from A/Califomia/04/2009 (H 1 N 1 ). The approximate concentration of the tissue
culture supernatant on the syringe needle was ~10A8 pfu/ml.
18:34 -A co-worker called the on-call iPhone, the lab manager answered, this co-worker relayed
that the researcher called out on the radio that help was needed because the researcher had stuck
themself with a needle in the ABSL-3+ laboratory. The co-worker was in the BSL-3Ag suite.
18:36 - The lab manager called the researcher in. The researcher had sprayed the puncture site
with disinfectant and had been running the site under water at the sink for 5 minutes. The lab
manager instructed the researcher to squeeze a few more drops of blood out of the finger and
continue running the site under water for another l O minutes, and then call back for the next set of
instructions.
18:43 - The lab manager asked the co-worker to listen for the radio in case the researcher had any
difficulty.
18 :46 - The lab manager called the ARO, relayed the situation, asks her to call the Infectious
Disease doctors.
18:54- The ARO called the UW Hospital Operator and asked to page the ID Fellow on-call.
18: 5 4 - The lab manager called the researcher and gave the researcher the following instructions:
put on new gloves, clean up the work area and then shower out normally. Do not hurry. Go
upstairs, sit in the conference room and do not leave the building.
18:57 - The lab manager called a senior scientist in the lab to call the researcher's family and
instruct them to start packing belongings to go to a hotel.
19: 19- ID Fellow called the ARO back and they discussed the situation. The Fellow paged the
attending to get the prescription sent in.
19:28- The ID attending physician called the ARO and got the required information for the
Tamiflu prescription.
I jRedacted by 19:29 - The lab manager called1agreement o report what happened.
19:40 - The lab manager arrived at the IRI, spoke with the researcher, and arranged for a hotel
3

room for the researcher's family.
20:10 -The lab manager called Walgreens to make sure the Tamiflu prescription was ready for
pickup and that Walgreens had the researcher's contact information and insurance information.
20: 15- The ID attending physician follows up with the ARO and asked for the lab manager's
contact information.
20: 18 - The ID Attending Physician called the lab manager to check that there are issues with
obtaining the Tamiflu prescription.
20:20 - Another co-worker went to pick up the prescription.
20:22- The ARO notified CDC DSAT via the Form 3 email address.
20:26-The ARO called the Director of University Health Services to explain the situation. The
Director sent an email to UW housing to see if we could get the a show apartment for the family
starting on Sunday.
20:31- The ARO called the City of Madison/Dane Public Health on-call pager.
20:32-The ARO called the Chief Medical Officer and State Epidemiologist for Wisconsin
Department of Health. He confinned the quarantine would be 7 days and the individual should be
taking a treatment dose of 75mg twice a day for 10 days.
20:34- The ARO talked to the City/Dane County Health on-call individuals and explains the
situation.
20:35pm -The research took the first Tamiflu dosage, (~2 hours after the injury). The Tamiflu
prescription was written for 75mg, once daily. (The lab manager voiced concern to the ARO about
the prescription being the prophylaxis dosage and not the treatment dosage. The ARO had already
spoken with the doctors about this, the Chief Medical Officer for Wisconsin Dept. of Public
Health wanted the researcher on the treatment dosage (75mg twice a day for 10 days). The
researcher was instructed to take one pill every 12 hours.
21 :00 - The researcher's family was pickup and driven to the hotel.
21 :30 - The researcher was driven home by the lab manager and was wearing a glove on the hand
with the puncture wound and an N~95 mask with no exhalation valve.
21 :00-22:00 - The lab manager spoke to two of the lab scientists about beginning the nasal and
throat swab sample testing the next morning.
21:55-Researcher took a reference body temperature: 98.1 F (axillary).
November 17. 2013
4

8:00 - The researcher too the second Tamiflu dosage and body temperature was 98.2 F (axillary).
9:00 - The lab manager obtained the first throat and nasal swab samples.
10:00 - Scientists began sample testing.
12:00 -The rapid Directigen test results are negative.
16:00 -The researcher's family was moved to a show apartment available on campus.
18:00 - The lab manager obtained nasal swab and throat gargle samples. The test results are
negative.
19:00 - The researcher's body temperature was 98.4 F (axillary).
November 18 2013
7: 15 - The researcher's temperature was 97.9F
9:00 - The researcher's body temperature was 98.96F (axillary). The UW-Madison Occuptional
Health nurse visited the researcher, drew blood, measured blood pressure and pulse (normal). The
lab manager obtained nasal swab and throat gargle samples. These test results were negative.
11:00-The researcher's body temperature is 99.IF (by mouth).
13:00 -The researcher's temperature is 98.6F (by mouth).
Daily updates are sent out by the ARO.
Has the IBC reviewed this incident? X No
If ves, orovide minutes
Has the root cause for this incident XYes
been identified? If yes, please describe: Employee failed to follow proper
procedure, despite training.
Describe measures taken by the institution to mitigate any problems identified. For measures identified but
not yet taken, please include a timeline for their implementation: (use additional space as necessary)
Employee will be retrained and SOPs will be rewritten to reflect a more direct and specific sharps policy.
NIH OBA Oct08
5
Bayha, Ryan (NIH/OD) [E]
From:
Sent:
To:
Cc
Subject:
!Redacted by agreement
Sunday, November 17, 2013 10;33 AM
Office of Biotechnology Activities (NIH/OD)
'rbayha@od.nih.gov'; 'kharris@od.nih.gov';""fe_,,d-act'""ed"'"'b-y-ag-re-em_e_,,.nt-------,
incident
A needle stick occurred last night, Saturday, November 16th, involving a non-transmissible strain of influenza HSN!. Toe
researcher immediately followed appropriate procedure. The ARO was contacted, followed by the RO, the CDC, myselfr
and City/County public health (not necessarily in that order). The researcher received his first dose of Tamiflu less than
two hours after the incident took place. Arrangements were immediately made to move the family of the researcher into
a model apartment on campus, with which we have an agreement, and to quarantine the researcher at home. PCR will
be run frequently to ensure immediate detection if it necessary. The Occupational medicine group on campus has also
been notified and will monitor/follow-up with the researcher.
This has been an exceptional response and everyone involved followed the procedures that have been put in place and
rehearsed regularly. I have limited details at this point as I was out of town at the time of the incident, but did still speak
with the ARO shortly after the incident. I will complete and submit a full report in the morning after being further briefed
by the ARO involved in the case, Please let me know if you have any questions in the mean time.
Redacted by agreement
Assistant Director
Biological Safety Officer
UW-EH&S
30 East Campus Mall
Madison, WI 53715
0 608 263-9013
C Redacted by agreement
http://www. e hs. wi sc.edu/bio-newsletter.htm

I
\
\
'
\
Template for Reporting Incidents Involving Recombinant DNA to the NIH
Office of Biotechnology Activities (OBA)
The Nil/ Guidelines for Research Involving Recombinant DNA Molecules (NIii Guidelines) states that" ... any
significant problems, violations of the NIH Guidelines, or any significant research-related accidents and
illnesses" must be reported to NIH OBA within 30 days. Certain types of incidents must be reported on a more
expedited basis. Spills or accidents in BSL-2 laboratories resulting in an overt exposure must be immediately
reported to NIH OBA. Spills or accidents occurring in high containment (BSL-3 or BSL-4) laboratories
resulting in an overt or potential exposure must be immediately reported to NIH OBA.
This template is intended to facilitate the reporting of incidents that occur during the conduct of research
subject to the NIH Guidelines. Use of this template 1s not required and other formats may be acceptable.
A separate template for reporting Human Gene Transfer Adverse Events is available at:
http://www4.od.nih.gov/oba/rac/adverse_event template.doc
Please note that submitting this completed template to the NIH OBA docs NOT fulfill the reporting
requirements of other agencies. You should verify with the other parties to whom you must report whether the
use of this template is acceptable.
Completed reports may be sent via [J.S. mail, courier service, e-mail, or facsimile to:
Attention: Incident Reports
NIH Office of Biotechnology Activities
6705 Rockledge Drive, Suite 750
Bethesda, Maryland 20892- 7985
(For all non-USPS deliveries use Zip Code 20817)
Telephone 301-496-9838
FAX 301-496-9839
E-mail: oba@od.nih.gov
--
NIH OBA Incident Rei!orting Teml!late
Does this incident involve research X Yes
subiect to the NIH Guidelines? If no, this incident does not have to be reeortcd to OBA
Institution name: University of Wisconsin - Madison
- - Date of report: 11/09/2013
Reporter name and position: Jim Turk Biological Safety Officer
Reporter telephone: (608)263-9013
Reporter email: jturk@fpm. wisc.cdu
----
Date of Incident: 11/09/2013
--
Name of principal investigator: Y oshihiro Kawaoka
---
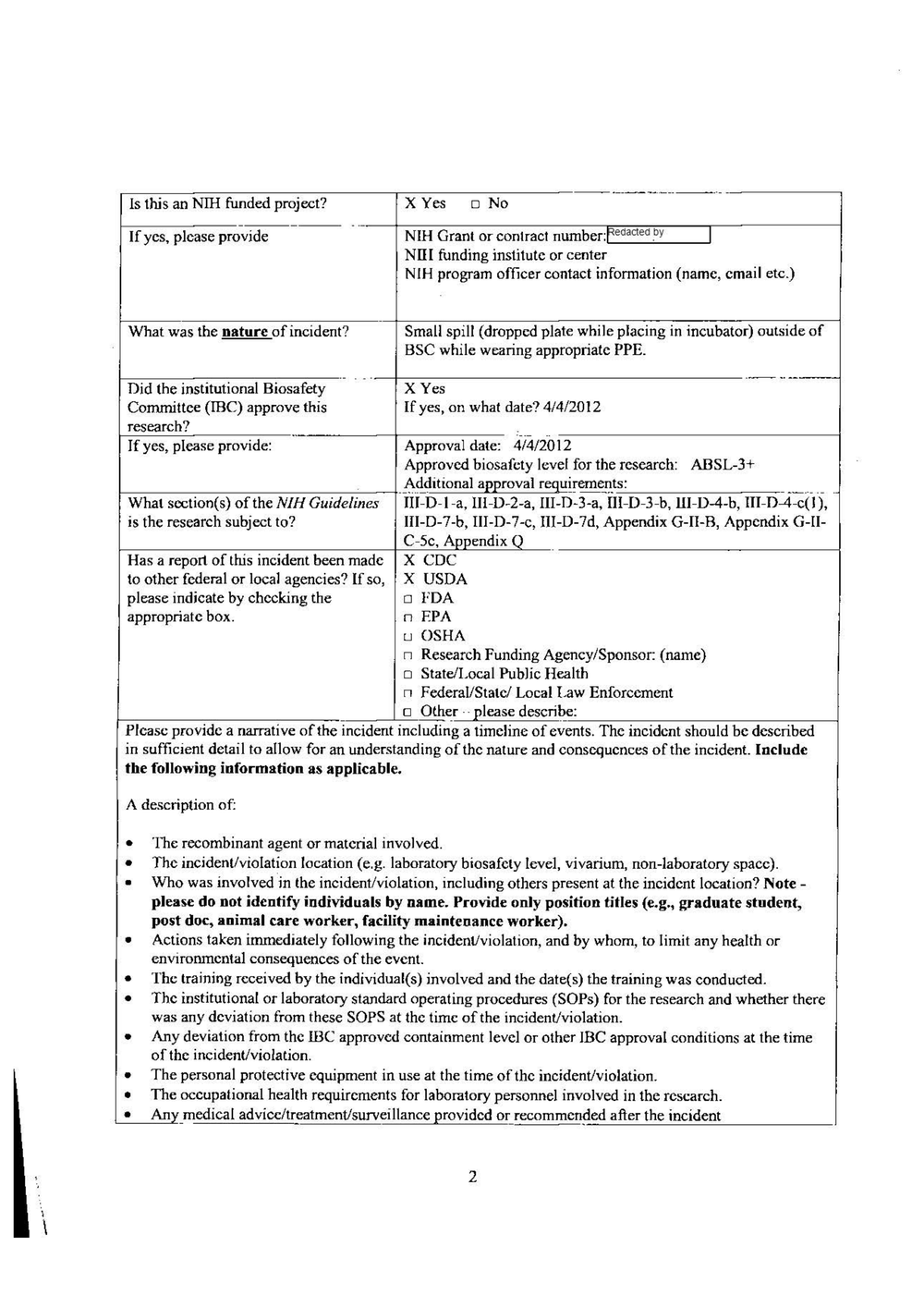
\
\
~-------------------.------------- ·--~----~-----·- ------~
ls this an NIH funded project?
If yes, please provide
What was the nature of incident?
X Yes o No
NIH Grant or contract number:fedacted _by I
NIii funding institute or center
NIH program officer contact information (name, email etc.)
Small spill (dropped plate while placing in incubator) outside of
BSC while wearing appropriate PPE.
t---------------. - ·--+-----------------------<o-••--
Did the institutional Biosafety X Yes
Committee (IBC) approve this If yes, on what date? 4/4/2012
research? 1-----------------------+-------
If yes, please provide: Approval date: 4/4/2012
Approved biosafcty level for the research: ABSL-3+
t------------------+ _ _bc!_ditional approval requirem~~_ts: _____ _
What scction(s) of the NIH Guidelines
is the research subject to?
Has a report of Lhis incident been made
to other federal or local agencies? lf so,
please indicate by checking the
appropriate box.
III-D-1-a lll-D-2-a III-D-3-a Ill-D-3-b lll-D-4-b III-D-4-c( l) ' , , ' ' , III-0- 7-b, III-D-7-c, III-D-7d, Appendix G-11-B, Appendix G-IIC-5c, Aooendix Q
X CDC
X USDA
o FDA
n EPA
u OSHA
n Research Funding Agency/Sponsor: (name)
o State/Local Public Health
n Federal/Statd Local Law Enforcement
--please describ_e_:_____________ ___,
Please provide a narrative of the incident including a timeline of events. The incident should be described
in sufficient detail to allow for an understanding of the nature and consequences of the incident. Include
the following information as applicable.
A description of:
• ]be recombinant agent or material involved.
• The incident/violation location (e.g. laboratory biosafcty level, vivarium, non-laboratory space).
• Who was involved in the incident/violation, including others present at the incident location? Note -
please do not identify individuals by name. Provide only position titles (e.g., graduate student,
post doc, animal care worker-, facility maintenance worker).
• Actions taken immediately following the incident/violation, and by whom, to limit any health or
environmental consequences of the event.
• The training received by the individual(s) involved and the date(s) the training was conducted.
• The institutional or laboratory standard operating procedures (SOPs) for the research and whether there
was any deviation from these SOPS at the time of the incident/violation.
• Any deviation from the IBC approved containment level or other JBC approval conditions at the time
of the incident/violation.
• The personal protective equipment in use at the time of the incident/violation.
• The occupational health requirements for laboratory personnel involved in the research.
• Any_medical advice/treatment/surveillance provided or recommcn~ed after the incident
2
• Any injury or illness associated with the incident.
• Medical surveillance results (if not available at the time of initial report please indicate when results
will be available).
• Eauioment failures.
DESCRIPTION OF INDICENT: (use additional space as necessary)
The researcher was wearing a PAPR, scrubs, tyvek, shoe covers, dedicated shoes,
another pair of shoe covers, and two pairs of gloves. All SOPs were followed
I appropriately and the incident is considered a spill and not an exposure. All personnel are 1 trained on a regular basis in accordance with the select agent regulations and go through
an extensive mentoring process.
This incident will be used as a training session for the laboratory. The group will discuss
what can be done to prevent a spill of this nature from occurring again. Mostly likely it will
be as simple as changing the way the plates are carried to the incubator.
Experiment set-up: The researcher was working in the ABSL-3+ suite performing growth
j curve analysis of viruses containing mutations in the PB2 protein (part of the viral
polymerase complex), in the virus strain background of A/Muscovy
Duck/Vietnam/TY93/2007 (H5N1: referred to as 'TY93'). The viral hemagglutinin (HA)
protein of this virus strain possesses a multi-basic cleavage site. Approximately 24 h prior
to the incident (on November 8th, 2013), cells in 6-well tissue culture plates were infected
at a multiplicity of 0.001 plaque forming units (PFU) per cell (~4 x 105 cells per well).
Following the infection, infected cells were covered with approximately 2 ml of media per
well, and cultures were incubated.
The spill: The spill occurred during the collection of supernatant samples from the
infected cultures at the 24 h time point (on the morning of November 9th, 2013). To
collect the virus culture supernatant samples, three 6-well tissue culture plates were
transferred by the researcher from the tissue culture incubator into a biosafety cabinet
(BSC), and a sample was harvested from each well into 2 ml screw-cap tubes. Following
sample collection, the researcher removed all three plates from the BSC (stacked on top
of each other) for transfer back into the tissue culture incubator. After opening the
I external door and the internal glass door of the incubator, the lower half of the tissue
culture plate on the bottom of the 3-plate stack slipped from the researcher's hand and
fell to the floor. Four wells of this plate were infected (2 wells each with two different virus
mutants: TY93-PB2-627K and TY93-PB2-627V), so approximately 8 ml of viruscontaining media spilled onto the floor.
Spill clean-up:
1. The researcher immediately closed the incubator doors and returned the plates that
were not dropped to the BSC. These plates were later transferred back to the tissue
culture incubator, following the spill clean-up procedure.
2. The researcher picked up the dropped plate bottom from the floor and immersed it in a
container of 5% MicroChem Plus inside the SSC.
3

3. The researcher then saturated his outer gloves with 70% ethanol, disposed of them in
the biohazard trash, and put on a new pair of outer gloves.
4. Following IRI SOP #33, the researcher covered the area of the spill (-3-inches in
diameter) with paper towels, and then flooded the contaminated area and paper towels
with freshly made 5% MicroChem Plus.
5. At this time, the researcher observed a few drops of liquid (i.e., media)~ek
suit below the knee, so 70% ethanol to saturate both arms (in entirety) and both legs
(from theknee aoWn) of the Tyvek suit, shoe covers, the bottoms of shoes, and 2-3
inches of exposed skin between the bottom of the Tyvek suit and the shoes (i.e., ankles).
6. After waiting 20 minutes, the researcher
a. Transferred the disinfectant-soaked paper towels covering the spill into a
biohazard autoclave bag inside a gray plastic bin.
b. Removed and disposed of his outer gloves into the same biohazard bag.
c. Donned a new pair of outer gloves.
d. Secured the biohazard bag by tying a knot at the top.
e. Sprayed the outside surface of the bag in 70% ethanQI.
7. The researcher prepared fresh 1 % Virkon S (from powder) and mopped the area of the
floor affected by the spill with 1 % Virkon S.
8. The researcher saturated the outside surfaces of the tissue culture incubators with
70% ethanol, and cleaned up the BSC according to standard procedures.
9. The researcher contacted the on-call scientist via the emergency iPhone to obtain
further instructions, and then exited the ABSL-3+ suite following the standard exit
procedure. Additional details about the incident response and communication are
described in Section IV below.
10. Following the researcher's exit from the ABSL-3+ suite, a second researcher entered
the suite and autoclaved out the disposable trash from the gown room, as well as all
trash inside the suite.
IV. Incident Response Communication and Timeline
1. 6:30 a.m. - The researcher spilled -8 ml of virus-containing media on the floor of the
ABSL-3+ suite (room 121 ), outside of BSC containment.
2. 6:30 - 6:54 a.m. - The researcher cleaned up the spill according to the steps
described in SOP #33.
3. 6:55 a.m. -The researcher phoned the on-call scientist via the emergency iPhone.
The researcher indicated to on-call scientist that a plate was dropped containing virus
onto the floor, and further indicated that was used SOP #33 to clean uo the soill. The on4

call scientist asked the researcher to await further instructions within the ABSL-3+ suite.
4. 7:09 a.m. - The on-call phone researcher phoned the lab manager to relay information
about the volume of the spill and to discuss how to proceed. Since the volume was close
to the amount considered to be a "large" spill (large spills are > 10 ml, and can be
considered a potential exposure), it was decided that additional consultation with the
Alternate Responsible Official (ARO) would be required before a decision about
quarantine could be made.
5. 7:15 a.m. - The on-call scientist phoned the ARO and left a message, describing the
incident and asking to phone back as soon as possible.
6. 7:21 a.m. -The on-call scientist notified the researcher that additional consultation
with the ARO was ongoing, and that the researcher should proceed to the conference
room, avoid contact with other people, and wait for further instructions.
7. 7:35 a.m. - The ARO returned the on-call scientist phone call. The situation was
summarized for the ARO.
8. 7:41 a.m. - The ARO called the researcher and they discussed the incident and the
strains being used. The ARO instructed the researcher to stay in the conference room
and while the necessary phone calls were made.
9. 7:52 a.m. - The ARO called the UW Hospital Operator and had the infectious disease
fellow on-call paged.
10. 7:55 a.m. - The on-call scientist notified the Principal Investigator about the incident
and the response up to this point.
11. 7:56 a.m. - The UW ID Fellow on-call, called the ARO. She explained the situation to
him and he consulted with his attending physician.
12. 8:12 a.m. -The ARO phoned to the on-call scientist to give a situational update
13. 8:23 a.m. -The Pl replied to the on-call scientist to indicate the receipt of the
information about the spill incident, and asked to be kept updated.
14. 8:28 a.m. - The UW ID Fellow called the ARO back and described that they would
not treat the individual due to the appropriate disinfection performed by researcher and
the risk of exposure through intact skin being very low. The ARO insisted however that
the researcher be given a Tamiflu prescription as a precaution as well as for peace of
mind. The UW ID Fellow agreed and called the researcher.
15. 8:31 a.m. - The ARO called the researcher and released the researcher from the
building. The ARO double checked the well-being of the researcher.
5
16. 8:34 a.m. -The ARO phoned the on-call scientist to relay the ID Consult team
decided to release the researcher without quarantine, and that there would be no need
for a Tamiflu prescription. However, as noted above, the researcher requested Tamiflu
anyway, and the UW ID Fellow stated that he would phone the researcher with the
prescription. The ID Consult Team also instructed that the researcher self-monitor for
any change in body temperature (every 12 h, at minimum) or feelings of illness, and The
ARO instructed the researcher that such observations must be communicated to the
ARO immediately upon observation.
17. The researcher took the first dose of Tamiflu in the afternoon, temperature remains
normal and is in good health.
Has the IBC reviewed this incident? X No
If yes, provide minutes
Has the root cause for this incident X Yes
been identified? If yes, please describe: Employee dropped plates while placing
in incubator.
Describe measures taken by the institution to mitigate any problems identified. For measures identified but
not yet taken, please include a timeline for their implementation: (use additional space as necessary)
The researcher was wearing a PAPR, scrubs, tyvek, shoe covers, dedicated shoes,
another pair of shoe covers, and two pairs of gloves. All SOPs were followed
appropriately and the incident is considered a spill and not an exposure. All personnel are
trained on a regular basis in accordance with the select agent regulations and go through
an extensive mentoring process.
This incident will be used as a training session for the laboratory. The group will discuss
what can be done to prevent a spill of this nature from occurring again. Mostly likely it will
be as simple as changing the way the plates are carried to the incubator.
Spill clean-up:
1. The researcher immediately closed the incubator doors and returned the plates that
were not dropped to the BSC. These plates were later transferred back to the tissue
culture incubator, following the spill clean-up procedure.
2. The researcher picked up the dropped plate bottom from the floor and immersed it in a
container of 5% MicroChem Plus inside the SSC.
3. The researcher then saturated his outer gloves with 70% ethanol, disposed of them in
the biohazard trash, and put on a new pair of outer gloves.
4. FollowinQ IRI SOP #33, the researcher covered the area of the soill /~3-inches in
6

diameter) with paper towels, and then flooded the contaminated area and paper towels
with freshly made 5% MicroChem Plus.
5. At this time, the researcher observed a few drops of liquid (i.e., media) on the Tyvek
suit below the knee, so 70% ethanol to saturate both arms (io entirety) and both legs
(from the knee down) of the Tyvek suit, shoe covers, the bottoms of shoes, and 2-3
inches of exposed skin between the bottom of the Tyvek suit and the shoes (i.e., ankles).
6. After waiting 20 minutes, the researcher
a. Transferred the disinfectant-soaked paper towels covering the spill into a
biohazard autoclave bag inside a gray plastic bin.
b. Removed and disposed of his outer gloves into the same biohazard bag.
c. Donned a new pair of outer gloves.
d. Secured the biohazard bag by tying a knot at the top.
e. Sprayed the outside surface of the bag in 70% ethanol.
7. The researcher prepared fresh 1 % Virkon S (from powder) and mopped the area of the
floor affected by the spill with 1% Virkon S.
8. The researcher saturated the outside surfaces of the tissue culture incubators with
70% ethanol, and cleaned up the SSC according to standard procedures.
9. The researcher contacted the on-call scientist via the emergency iPhone to obtain
further instructions, and then exited the ABSL-3+ suite following the standard exit
procedure. Additional details about the incident response and communication are
described in Section IV below.
10. Following the researcher's exit from the ABSL-3+ suite, a second researcher entered
the suite and autoclaved out the disposable trash from the gown room, as well as all
trash inside the suite.
IV. Incident Response Communication and Timeline
1. 6:30 a.m. - The researcher spilled -8 ml of virus-containing media on the floor of the
ABSL-3+ suite (room 121 ), outside of BSC containment.
2. 6:30 - 6:54 a.m. - The researcher cleaned up the spill according to the steps
described in SOP #33.
3. 6:55 a.m. -The researcher phoned the on-call scientist via the emergency iPhone.
The researcher indicated to on-call scientist that a plate was dropped containing virus
onto the floor, and further indicated that was used SOP #33 to clean up the spill. The oncall scientist asked the researcher to await further instructions within the ABSL-3+ suite.
4. 7:09 a.m. - The on-call phone researcher phoned the lab manager to relay information
about the volume of the soill and to discuss how to oroceed. Since the volume was close
7

to the amount considered to be a "large" spill (large spills are > 10 ml, and can be
considered a potential exposure), it was decided that additional consultation with the
Alternate Responsible Official (ARO) would be required before a decision about
quarantine could be made.
5. 7:15 a.m. - The on-call scientist phoned the ARO and left a message, describing the
incident and asking to phone back as soon as possible.
6. 7:21 a.m. -The on-call scientist notified the researcher that additional consultation
with the ARO was ongoing, and that the researcher should proceed to the conference
room, avoid contact with other people, and wait for further instructions.
7. 7:35 a.m. - The ARO returned the on-call scientist phone call. The situation was
summarized for the ARO.
8. 7:41 a.m. - The ARO called the researcher and they discussed the incident and the
strains being used. The ARO instructed the researcher to stay in the conference room
and while the necessary phone calls were made.
9. 7:52 a.m. - The ARO called the UW Hospital Operator and had the infectious disease
fellow on-call paged.
10. 7:55 a.m. - The on-call scientist notified the Principal Investigator about the incident
and the response up to this point
11. 7:56 a.m. - The UW ID Fellow on-call, called the ARO. She explained the situation to
him and he consulted with his attending physician.
12. 8:12 a.m. - The ARO phoned to the on-call scientist to give a situational update
13. 8:23 a.m. - The Pl replied to the on-call scientist to indicate the receipt of the
information about the spill incident, .and asked to be kept updated.
14. 8:28 a.m. - The UW ID Fellow called the ARO back and described that they would
not treat the individual due to the appropriate disinfection performed by researcher and
the risk of exposure through intact skin being very low. The ARO insisted however that
the researcher be given a Tamiflu prescription as a precaution as well as for peace of
mind. The UW ID Fellow agreed and called the researcher.
15. 8:31 a.m. - The ARO called the researcher and released the researcher from the
building. The ARO double checked the well-being of the researcher.
16. 8:34 a.m. -The ARO phoned the on-call scientist to relay the ID Consult team
decided to release the researcher without quarantine, and that there would be no need
for a Tamiflu prescription. However, as noted above, the researcher requested Tamiflu
an_yv,,a , and the UW ID Fellow stated that he would hone the researcher with the
8

prescription. The ID Consult Team also instructed that the researcher self"'.monitor for
any change in body temperature (every 12 h, at minimum) or feelings of illness, and The
ARO instructed the researcher that such observations must be communicated to the
ARO immediately upon observation.
17. The researcher took the first dose of Tamiflu in the afternoon, temperature remains
normal and is in good health.
NIH OBA Oct08
9