Documents
Chikungunya Lab Infection Report to NIH
Nov. 1 2022 — 11:45 a.m.

October 28, 20 16
Ph.D., CBSP
Director, Office of Biological and Chemical Safety
Environmental Hea lth and Safety
Washington University
660 South Euclid Avenue
Campus Box 8229
St. Louis, MO 63 I I 0
Dear Dr.
U.S. Public Health Service
Bethesda, Maryland 20892
Office of Science Policy
National Institutes of Health
6705 Rockledge Drive
Suite 750. MSC 7985
Bethesda. MD 20892-7985
(301 ) 496-9838 (Phone)
(301) 496-9839 (Fax)
http://osp.od . nih.gov
Thank you for your September 30, 20 16, and October 13. 2016, rep01ts to the Nationa l Insti tutes of Health
(NIH) Office of Science Poli cy (OSP) regarding a September 24. 2016. incident in wh ich a grad uate student
grazed her hand with a needle while administering an antibody to mice in fected with Chikun gunya virus
(CHIKV) in a biosafety level (BL) 3 laboratory at Washington Un iversity.
From your report, we understand that the student grazed her fi nger while hand ling the need le after
perfom1ing an injection. She was wearing appropriate persona l protective equ ipment (PPE). inc luding
double gloves, at the time of the incident, but the needle broke through both pa irs of gloves. She
immediately washed her hands with soap and water.
On September 28. 20 16, the student deve loped a fever with severe body ac hes. On September 29, 20 16. she
presented with a mac ular rash which worsened throughout the day. That evening she reported her
symptoms and the needle stick to the principal investigator (PI) and went to the hospital. She was kept in
hospital overnight fo r observation, and the followi ng day was seen by an infect ion disease specia list who
sent blood to the state laboratories for CHIKV testing. She was released fi·om hospita l that day.
By October 2, 201 6, the feve r and rash had gone and the student did not deve lop arthralgia or a11hritis.
\vhich is often assoc iated with Chikungunya fever. However. she did rece ive positive CH IKV qPCR res ults
from the Infectious Disease clin ic.
In response to this incident, the PI met with al l laboratory personne l to disc uss the proper repo11 ing of
personnel exposures and the processes in place for reporting incidents. The Department of Env ironmenta l
Health and Safety will add additional slides about sharps safety to the annua l laboratory tra ining.
No ftnther information about this incident is required at this time. However. we are concerned that an
exposure inc ident occ urred in a BL3 laboratory and \vent unreported for four days. We recommend that the
requirement to immediately report any overt or potential exposure be stressed to all personnel work ing in
high containment. We also recommend eva luating exactly how the injury occurred, incl uding how the
Name
Name

Ph.D., CBSP
October 28, 2016
Page 2
student was handling the needle after conducting the injection. The student may require additional hands on
training on how to safely handle sharps. Needles should be discarded in an appropriate sharps container
immediately after use to minimize the potential for a stick. The use of safety needles. where the needle can
be sheathed immediately after use should also be considered. Please contact Dr. Kathryn Han·is, Senior
Outreach and Education Specialist, by email at harriskath@od.nih.gov or by telephone at (30 I) 496-9838 if
you have any additional questions.
Sincerely,
lfi )c~~ Christopher Viggiani, Ph.D.
Program Director, Biosafety and Biosecurity Policy
Biosafety, Biosecurity and Emerging Biotechnology
Policy Division, NIH
cc: Carrie D. Wolinetz, Ph .D., Associate Director !'or Science Policy. NIH
Jessica Tucker. Ph .D .. Director. Biosafety. Biosecurity and Emerging Biotechnology Policy
Division. NIH
Kathryn Harris, Ph.D .. RBP, Senior Outreach and Education Specialist (contractor), Biosafety,
Biosecurity and Emerging Biotechnology Policy Division, NIH
Laura Cochran, Program Assistant (contractor), Biosafety, Biosecurity and Emerging
Biotechnology Policy Division, NIH
Name
Harris, Kathryn (NIH/00) [C]
From:
Sent:
To:
Subject:
Attachments:
Kathryn,
@wustl.edu >
Thursday, October 13, 2016 5:17 PM
Harris, Kathryn (NIH/00) [C]
RE: Report of potential exposure
Incident Report Follow Up 161013.pdf
Attached, please find the completed final report for this exposure. Please let me know if I can provide any additional information.
-Susan
PhD, CBSP
Director, Office of Biological & Chemical Safety
Environmental Health & Safety
Campus Box 8229
Phone:3 14-747-03 09;Fax: 314-362-6786
Email: @wustl.edu; Web: ehs.wustl.edu
From: Harris, Kathryn (NIH/OD) [C] [mailto:HarrisKath@mail.nih.gov]
Sent: Friday, October 07, 2016 8:40AM
To:
Subject: RE: Report of potential exposure
Dea r
Thanks for the update. It would be helpful if you could compile a formal final report regarding the event, immediate
actions taken and actions to prevent similar accidents. Our new incident reporting template articu lating the kinds of
informat ion a report should contain is available at
http://osp.od.nih.gov/sites/default/files/resources/lncident%20Reporting%20Template%20-%202016 2.docx
Thanks,
Ka t hryn
From: [mailto: @wustl.edu]
Sent: Friday, October 07, 2016 9:33AM
To: NIH guidelines <NIHguidelines@od .nih.gov>
Cc: @wustl.edu>
Subject: RE: Report of potentia l exposure
Yesterday afternoon, the P1 contacted me to let me know that the student involved has recovered and is back at work. The student
never developed arthritis or joint swelling but the PCR results for Chikungunya were positive. Now that the student is recovered, I
have asked the PI to talk with her to get more details on the circumstances surrounding the incident and what can be done to prevent
similar incidents in the future. 1 have also stressed the importance of reporting any exposures or potential exposures immediately. I
will pass along any additional information as it becomes available but please let me know if there are other items you would like
addressed.
Name
Name
Name
Name
Name
Name Name
Name
Name

PhD, CBSP
Director, Office of Biological & Chemical Safety
Environmental Health & Safety
Campus Box 8229
Phone : 314-747-03 09; Fax: 314-362-6786
Email: @wustl.edu; Web: ehs.wustl.edu
From:
Sent: Friday, September 30, 2016 6:55 PM
To: NIHGuidelines@od.nih.gov
Cc:
Subject: Report of potential exposure
I am writing to report a potential exposure to recombinant DNA. On September 30th, I was contacted by a principal investigator
whose graduate student had been admitted to the ER on the night of September 29th with high fever, muscle aches, and rash. On the
30th, the student told the PI that on September 24th she had sustained a scratch with a needle used to inject antibodies into a mouse
that had been previously infected with a recombinant clone ofChikungunya virus (Ia Reunion 2006 strain). The student did not see
any blood from the scratch so she did not report it or seek medical attention. The student has not received a diagnosis yet but the
Infectious Diseases division of the hospital as well as Occupational and Student Health have been contacted for consultations.
At this time, I do not have any additional details. I am gathering information about the circumstances surrounding the exposure as
well as the student's current condition and diagnosis and will pass that information along as soon as I can. The Co-Chairs of the IBC
have been notified of this situation and the full committee will discuss the potential exposure at the next scheduled IBC meeting on
October 19th.
I will be out of the office for the ABSA conference next week but will be checking email frequently to monitor this situation. I'm also
copying Associate Biological & Chemical Safety Officer, who will be able to assist you in my absence.
-Susan
PhD, CBSP
Biological & Chemical Safety Officer, Washington University
660 South Euclid A venue, Campus Box 8229
St. Louis, MO 63110
Phone (314) 747-0309; Fax (314) 362-6786
Email: @wustl.edu; Web: http://ehs.wustl.edu
2
Name
Name
Name
Name
Name
Name
Name

Template for Reporting Incidents Subject to the
NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid
Molecules to the National Institutes of Health
Office of Science Policy (OSP)
IlPPI\.\. National Institutes of Health / Office of Science Policy
Web: http//osp.od.nih.gov
Address : 6705 Rockledge Dr #750. Bethesda. MD 20817
Phone: (301) 496-9838

~~1 ~ ~q:~~~~rz:;v ·~~ ·· ~. -. -,-~17j"f~,~:-·
~~ '""" : ~t"" . h.:: •
~J·-~~ ~ ~-
Instructions for Completing this Template
The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid
Molecules (NIH Guidelines) states that " ... any significant problems, violations of the NIH
Guidelines, or any significant research-related accidents and illnesses" must be
reported to NIH within 30 days. Certain types of incidents must be reported on a more
expedited basis. Spills or accidents occurring in Biosafety Level (BL) 2 laboratories
resulting in an overt exposure must be immediately reported to NIH. Spills or accidents
occurring in high containment (BL3 or BL4) laboratories resulting in an overt or potential
exposure must be immediately reported to NIH.
This template is intended to facilitate the reporting of incidents that occur during the
conduct of research subject to the NIH Guidelines. Please complete all fields as fully as
possible. The use of this template is not required and other formats for submitting
reports may be acceptable.
Completed reports may be sent to OSP via email at NIHGuidelines@od.nih.qov
Please Note:
Human Gene Transfer (HGT) Adverse Events (AEs) should still be reported to the NIH
Office of Science Policy (OSP).
A separate template for reporting Human Gene Transfer Adverse Events is available
here.
HGT AEs should be emailed to HGTprotocols@mail.nih .gov
2
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy
Does this incident involve [3JYES DNO research subject to the NIH
Guidelines?
If no, this incident does not require reporting to OSP
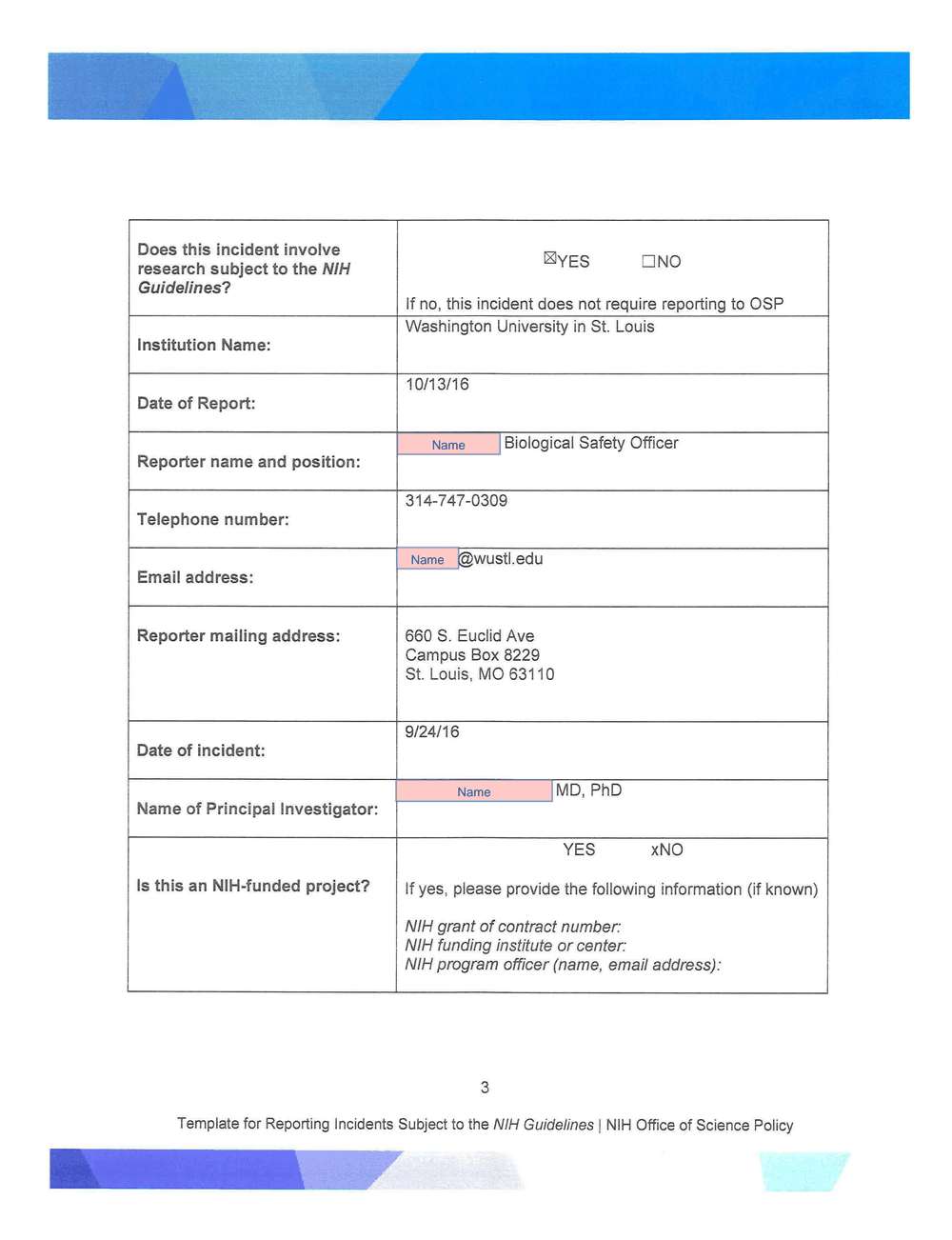
Washington University in St. Louis
Institution Name:
10/13/16
Date of Report:
Biological Safety Officer
Reporter name and position:
314-7 4 7-0309
Telephone number:
@wustl.edu
Email address:
Reporter mailing address: 660 S. Euclid Ave
Campus Box 8229
St. Louis, MO 63110
9/24/16
Date of incident:
MD, PhD
Name of Principal Investigator:
YES xNO
Is this an NIH-funded project? If yes, please provide the following information (if known)
NIH grant of contract number:
NIH funding institute or center:
NIH program officer (name, email address):
3
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy
~ ~:r-t!'r-""'·:. ·~., , ••. -;~- --------~~~-~~~~ 1~ ~ - 1 " . ~ . ,, -~
~:,. ~ • , , • ~ f I ' :•..1 _ ,,._, I -
Name
Name
Name
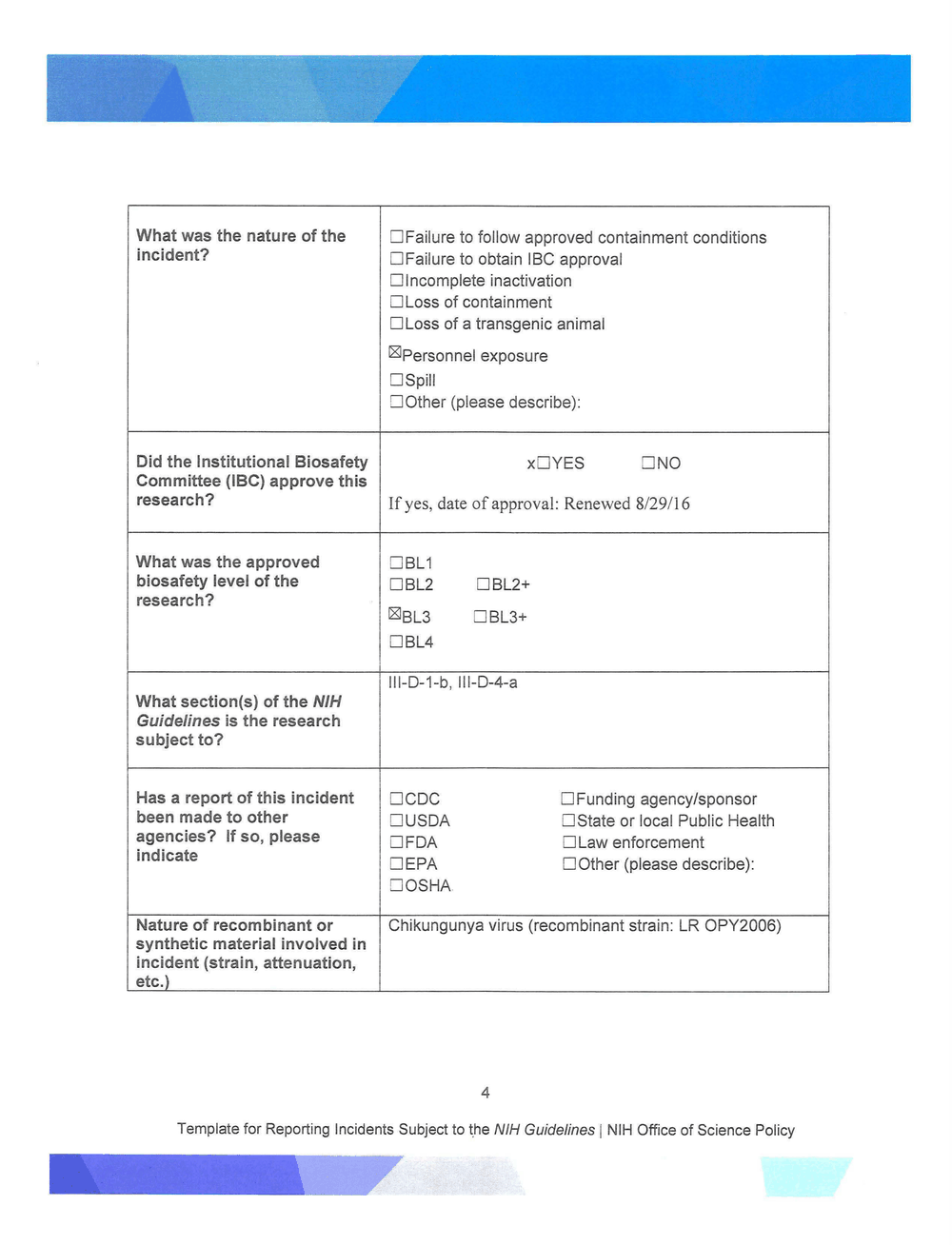
What was the nature of the
incident?
Did the Institutional Biosafety
Committee (IBC) approve this
research?
What was the approved
biosafety level of the
research?
What section(s) of the NIH
Guidelines is the research
subject to?
Has a report of this incident
been made to other
agencies? If so, please
indicate
DFailure to follow approved containment conditions
DFailure to obtain IBC approval
D Incomplete inactivation
D Loss of containment
DLoss of a transgenic animal
~Personnel exposure
DSpill
DOther (please describe):
xDYES DNO
If yes, date of approval: Renewed 8/29/l6
DBL1
DBL2
~BL3
DBL4
DBL2+
DBL3+
111 -D-1-b, 111 -D-4-a
DCDC
DUSDA
DFDA
DEPA
DOSHA
D Funding agency/sponsor
D State or local Public Health
D Law enforcement
DOther (please describe):
Nature of recombinant or Chikungunya virus (recombinant strain: LR OPY2006)
synthetic material involved in
incident (strain, attenuation,
etc.)
4
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy
.. f'="'• .,., ........ -~·~-::~;,"=.-- .. ~(?t:",:':'r.~.
• -~ ~ t '.,.,~ '"'~~~
\ ~ ...

Please provide a narrative of the incident including a timeline of events. The incident should be
described in sufficient detail to allow for an understanding of the nature and consequences of
the incident. Include the following information as applicable.
A description of:
• The incident/violation location (e.g. laboratory biosafety level, vivarium, non-laboratory
space)
• Who was involved in the incident/violation, including others present at the incident
location?
Note - please do not identify individuals by name. Provide only gender and
position titles (e.g., graduate student, post doc, animal care worker, facility
maintenance worker)
• Actions taken immediately following the incident/violation, and by whom, to limit any
health or environmental consequences of the event
• The training received by the individual(s) involved and the date(s) the training was
conducted
• The institutional or laboratory standard operating procedures (SOPs) for the research
and whether there was any deviation from these SOPS at the time of the
incident/violation
• Any deviation from the IBC approved containment level or other IBC approval conditions
at the time of the incident/violation
• The personal protective equipment in use at the time of the incident/violation
• The occupational health requirements for laboratory personnel involved in the research
• Any medical surveillance provided or recommended after the incident
• Any injury or illness associated with the incident
• Equipment failures
5
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy
" - . --: ~- .,:;·1>.·: _ .. ~ ~- :~;-;~ ' 0 • ~ ~ 0 r • ~~
' "' ~ .. '' § : .. ~ -~~~: . .'14:~;l,:.:~·

,~~ ·~7·:·':;;-~.~ ·::""''':'"0:'
. ' '... · .. - ~.... .
J ~--
~ .. J •
DESCRIPTION OF INCIDENT: (use additional space as necessary)
The incident took place in our ABSL3 laboratory space (inside a biosafety cabinet) on
Saturday 9/24/16. A graduate student was administering antibody to mice infected with
CHIKV-LR2006 strain via an intraperitoneal route at a time point where infectious virus
would still be present in the mice. There were no other lab members present at the time,
and proper BSL3 personal protective equipment was in use at the time of the incident
(double gowns, double gloves, double shoe covers, hair net, and face mask). All lab
personnel that use the BSL3 space have had proper training in accordance with
national and institutional guidelines.
She grazed a finger on her left hand while handling the needle after an IP injection. The
needle did break through both sets of gloves (by visible inspection), but there was no
visible sign of blood (i.e. it didn't seem like a deep needle stick). The student washed
her hands with soap and water immediately following the needle stick.
Wednesday evening (9/28/16 - 4 days post needle stick) the graduate student started
running a fever and had severe body aches and chills. Thursday morning (9/29/16) she
presented with a macular rash, which progressively worsened throughout the day.
Thursday evening she reported her symptoms and needle stick incident to her PI and
checked into the ER for treatment. She was kept overnight for observation and was
given ibuprofen for the fever and pain and fluids for hydration. On Friday 9/30/16 she
was seen by the Infectious Disease consult service and blood was sent to the state labs
for CHIKV tests. She was released from the hospital that Friday afternoon.
By Sunday (1 0/2/16) her rash and fever were gone, and she was back at work on
Tuesday 10/4/16. Ultimately, she experienced only 3 days of more severe symptoms,
and she never developed the arthralgia and arthritis often associated with chikungunya
fever. On Thursday 10/6/16 she did receive positive CH IKV qPCR results from the
Infectious Disease clinic.
6
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy
-;..;-::- - .. - ~~~.., ...... ,];!- :-:--:-- ~..,.; :r-:--1 .i-- ~ ~·l
' 1 ~ /j I <"'

-.·~~or"' -:-... ~.~. .. "(" .. < : •<
~~ ~- ~
> ••
""'""' >
r -~ •
Has the IBC reviewed this xYES DNO
incident?
Accident
Please describe the root cause
of this incident:
Describe measures taken by the institution to mitigate any problems identified. For measures
identified but not yet taken, please include a timeline for their implementation (use additional
space as necessary):
Prior to this incident, EH&S distributed a sharps safety reminder through the University's
Research News tool. The day prior to the incident, EH&S sent annual safety and compliance
reports to all department chairs and business managers in departments that have laboratories
and clinics. The report highlighted needle stick trends, asking departments to focus on reducing
needle sticks and other common injuries, and asked department chairs to pay special attention
to prompt reporting of any potential exposure to recombinant DNA-containing material, as
required by NIH. Following this incident, the PI discussed proper reporting of personnel
exposure in a lab meeting with all members of the lab and procedures in place for reporting
such incidents were reviewed with all members of the lab being present. EH&S will also add
additional slides about sharps safety to the annual laboratory safety training . The IBC will
discuss at the October meeting what other educational steps can be taken to minimize the
chance of sharps injuries in the future.
• Additional information may be requested by NIH OSP after review of this report
depending on the nature of the incident.
7
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy

• Submitting this completed template to NIH OSP does NOT fulfill the reporting
requirements of other agencies. You should verify with the other parties to whom
you must report whether the use of this template is acceptable.
8
Template for Reporting Incidents Subject to the NIH Guidelines 1 NIH Office of Science Policy

Harris, Kathryn (NIH/OD) [C]
From:
Sent:
To:
@wustl.edu>
Friday, October 07, 2016 9:33 AM
NIH guidelines
Cc:
Subject: RE: Report of potential exposure
Yesterday afternoon, the PI contacted me to let me know that the student involved has recovered and is back at work. The student
never developed arthritis or joint swelling but the PCR results for Chikungunya were positive. Now that the student is recovered, I
have asked the PI to ta lk with her to get more details on the circumstances surrounding the incident and what can be done to prevent
similar incidents in the future . I have also stressed the importance of reporting any exposures or potential exposures immediately. I
will pass along any additional information as it becomes available but please let me know if there are other items you would like
addressed.
PhD, CBSP
Director, Office of Biological & Chemical Safety
Environmental Health & Safety
Campus Box 8229
Phone: 314-747-0309; Fax: 314-362-6786
Email: @wustl.edu; Web: ehs.wustl.edu
From:
Sent: Friday, September 30, 2016 6:55 PM
To: NIHGuidelines@od.nih.gov
Cc:
Subject: Report of potential exposure
I am writing to report a potential exposure to recombinant DNA. On September 30th, I was contacted by a principal investigator
whose graduate student had been admitted to the ER on the night of September 29th with high fever, muscle aches, and rash. On the
30th, the student told the PI that on September 24th she had sustained a scratch with a needle used to inject antibodies into a mouse
that had been previously infected with a recombinant clone of Chikungunya virus (la Reunion 2006 strain). The student did not see
any blood from the scratch so she did not report it or seek medical attention. The student has not received a diagnosis yet but the
Infectious Diseases division of the hospital as well as Occupational and Student Health have been contacted for consultations.
At this time, I do not have any additional details. I am gathering information about the circumstances surrounding the exposure as
well as the student's current condition and diagnosis and will pass that information along as soon as I can. The Co-Chairs ofthe IBC
have been notified of this situation and the full committee will discuss the potential exposure at the next scheduled IBC meeting on
October 19th.
I will be out of the office for the ABSA conference next week but will be checking email frequently to monitor this situation. I'm also
copying Associate Biological & Chemical Safety Officer, who will be able to assist you in my absence.
PhD, CBSP
Biological & Chemical Safety Officer, Washington University
660 South Euclid Avenue, Campus Box 8229
St. Louis, MO 63110
Phone (314) 747-0309; Fax (314) 362-6786
Email: @wustl.edu; Web: http://ehs.wustl.edu
1
Name
Name
Name
Name
Name
Name
Name
Name
Name
Name
Name

Harris, Kathryn (NIH/OD) [C]
From:
Sent:
To:
Cc:
Subject:
@wustl.edu>
Friday, September 30, 2016 7:55 PM
NIH guidelines
Report of potential exposure
I am writing to report a potential exposure to recombinant DNA. On September 30th, I was contacted by a principal investigator
whose graduate student had been admitted to the ER on the night of September 29th with high fever, muscle aches, and rash. On the
30th, the student told the PI that on September 24th she had sustained a scratch with a needle used to inject antibodies into a mouse
that had been previously infected with a recombinant clone of Chikungunya virus (Ia Reunion 2006 strain). The student did not see
any blood from the scratch so she did not report it or seek medical attention. The student has not received a diagnosis yet but the
Infectious Diseases division of the hospital as well as Occupational and Student Health have been contacted for consultations.
At this time, I do not have any additional details. I am gathering information about the circumstances surrounding the exposure as
well as the student's current condition and diagnosis and will pass that information along as soon as I can. The Co-Chairs of the IBC
have been notified of this situation and the full committee will discuss the potential exposure at the next scheduled IBC meeting on
October 19th.
I will be out of the office for the ABSA conference next week but will be checking email frequently to monitor this situation. I'm also
copying Associate Biological & Chemical Safety Officer, who will be able to assist you in my absence.
PhD, CBSP
Biological & Chemical Safety Officer, Washington University
660 South Euclid A venue, Campus Box 8229
St. Louis, MO 63110
Phone (314) 747-0309; Fax (314) 362-6786
Email: @wustl.edu; Web: http://ehs.wustl.edu
Name
Name
Name
Name
Name
Name